A Novel Role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma
- PMID: 28042859
- PMCID: PMC6155798
- DOI: 10.3390/molecules22010062
A Novel Role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma
Abstract
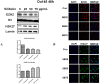
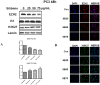
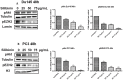
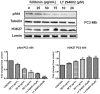
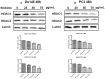
Silibinin, extracted from milk thistle (Silybum marianum L.), has exhibited considerable preclinical activity against prostate carcinoma. Its antitumor and chemopreventive activities have been associated with diverse effects on cell cycle, apoptosis, and receptor-dependent mitogenic signaling pathways. Here we hypothesized that silibinin's pleiotropic effects may reflect its interference with epigenetic mechanisms in human prostate cancer cells. More specifically, we have demonstrated that silibinin reduces gene expression levels of the Polycomb Repressive Complex 2 (PRC2) members Enhancer of Zeste Homolog 2 (EZH2), Suppressor of Zeste Homolog 12 (SUZ12), and Embryonic Ectoderm Development (EED) in DU145 and PC3 human prostate cancer cells, as evidenced by Real Time Polymerase Chain Reaction (RT-PCR). Furthermore immunoblot and immunofluorescence analysis revealed that silibinin-mediated reduction of EZH2 levels was accompanied by an increase in trimethylation of histone H3 on lysine (Κ)-27 residue (H3K27me3) levels and that such response was, in part, dependent on decreased expression levels of phosphorylated Akt (ser473) (pAkt) and phosphorylated EZH2 (ser21) (pEZH2). Additionally silibinin exerted other epigenetic effects involving an increase in total DNA methyltransferase (DNMT) activity while it decreased histone deacetylases 1-2 (HDACs1-2) expression levels. We conclude that silibinin induces epigenetic alterations in human prostate cancer cells, suggesting that subsequent disruptions of central processes in chromatin conformation may account for some of its diverse anticancer effects.
Keywords: DNMT; EZH2; H3K27me3; HDAC; PRC2; epigenetics; histone methylation; prostate cancer; silibinin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

