Effects of VPAC1 activation in nucleus ambiguus neurons
- PMID: 28043808
- PMCID: PMC5303116
- DOI: 10.1016/j.brainres.2016.12.026
Effects of VPAC1 activation in nucleus ambiguus neurons
Abstract
The pituitary adenylyl cyclase-activating polypeptide (PACAP) and its G protein-coupled receptors, PAC1, VPAC1 and VPAC2 form a system involved in a variety of biological processes. Although some sympathetic stimulatory effects of this system have been reported, its central cardiovascular regulatory properties are poorly characterized. VPAC1 receptors are expressed in the nucleus ambiguus (nAmb), a key center controlling cardiac parasympathetic tone. In this study, we report that selective VPAC1 activation in rhodamine-labeled cardiac vagal preganglionic neurons of the rat nAmb produces inositol 1,4,5-trisphosphate receptor-mediated Ca2+ mobilization, membrane depolarization and activation of P/Q-type Ca2+ channels. In vivo, this pathway converges onto transient reduction in heart rate of conscious rats. Therefore we demonstrate a VPAC1-dependent mechanism in the central parasympathetic regulation of the heart rate, adding to the complexity of PACAP-mediated cardiovascular modulation.
Keywords: Autonomic cardiac tone; Bradycardia; Calcium signaling; PACAP.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
statement All authors declare that there are no conflicts of interest.
Figures



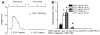

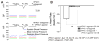

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

