IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis
- PMID: 28043870
- PMCID: PMC5494022
- DOI: 10.1016/j.jaci.2016.08.056
IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis
Abstract
Background: Generalized pustular psoriasis (GPP) is a rare, debilitating, and often life-threatening inflammatory disease characterized by episodic infiltration of neutrophils into the skin, pustule development, and systemic inflammation, which can manifest in the presence or absence of chronic plaque psoriasis (PV). Current treatments are unsatisfactory and warrant a better understanding of GPP pathogenesis.
Objective: We sought to understand better the disease mechanism of GPP to allow improved targeted therapies.
Methods: We performed a gene expression study on formalin-fixed paraffin-embedded GPP (n = 28) and PV (n = 12) lesional biopsies and healthy control (n = 20) skin. Differential gene expression was analyzed using gene ontology and enrichment analysis. Gene expression was validated with quantitative RT-PCR and immunohistochemistry, and a potential disease mechanism was investigated using primary human cell culture.
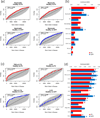
Results: Compared with healthy skin, GPP lesions yielded 479 and PV 854 differentially expressed genes, respectively, with 184 upregulated in both diseases. We detected significant contributions of IL-17A, TNF, IL-1, IL-36, and interferons in both diseases; although GPP lesions furnished higher IL-1 and IL-36 and lower IL-17A and IFN-γ mRNA expression than PV lesions did. We detected prominent IL-36 expression by keratinocytes proximal to neutrophilic pustules, and we show that both neutrophils and neutrophil proteases activate IL-36. Suggesting another mechanism regulating IL-36 activity, the protease inhibitors serpin A1 and A3, which inhibit elastase and cathepsin G, respectively, were upregulated in both diseases and inhibited activation of IL-36.
Conclusions: Our data indicate sustained activation of IL-1 and IL-36 in GPP, inducing neutrophil chemokine expression, infiltration, and pustule formation, suggesting that the IL-1/IL-36 inflammatory axis is a potent driver of disease pathology in GPP.
Keywords: Generalized pustular psoriasis; inflammation; interleukin; psoriasis.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Gudjonsson JE, Elder JT. Chapter 18. Psoriasis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJKW, editors. Fitzpatrick’s dermatology in general medicine. 1. 8th. New York: McGraw-Hill Medical; 2012.
-
- Hussain S, Berki DM, Choon SE, Burden AD, Allen MH, Arostegui JI, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135(4):1067–1070. e9. - PubMed
-
- Setta-Kaffetzi N, Navarini AA, Patel VM, Pullabhatla V, Pink AE, Choon SE, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133(5):1366–1369. - PubMed
-
- Sugiura K, Takemoto A, Yamaguchi M, Takahashi H, Shoda Y, Mitsuma T, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133(11):2514–2521. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

