Integrin alpha6 maintains the structural integrity of the kidney collecting system
- PMID: 28043890
- PMCID: PMC5330664
- DOI: 10.1016/j.matbio.2016.12.003
Integrin alpha6 maintains the structural integrity of the kidney collecting system
Abstract
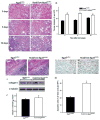
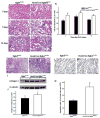
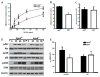
Laminins are a major constituent of the basement membranes of the kidney collecting system. Integrins, transmembrane receptors formed by non-covalently bound α and β subunits, serve as laminin receptors, but their role in development and homeostasis of the kidney collecting system is poorly defined. Integrin α3β1, one of the major laminin receptors, plays a minor role in kidney collecting system development, while the role of α6 containing integrins (α6β1 and α6β4), the other major laminin receptors, is unknown. Patients with mutations in α6 containing integrins not only develop epidermolysis bullosa, but also have abnormalities in the kidney collecting system. In this study, we show that selectively deleting the α6 or β4 integrin subunits at the initiation of ureteric bud development in mice does not affect morphogenesis. However, the collecting system becomes dilated and dysmorphic as the mice age. The collecting system in both null genotypes was also highly susceptible to unilateral ureteric obstruction injury with evidence of excessive tubule dilatation and epithelial cell apoptosis. Mechanistically, integrin α6-null collecting duct cells are unable to withstand high mechanical force when adhered to laminin. Thus, we conclude that α6 integrins are important for maintaining the integrity of the kidney collecting system by enhancing tight adhesion of the epithelial cells to the basement membrane. These data give a mechanistic explanation for the association between kidney collecting system abnormalities in patients and epidermolysis bullosa.
Copyright © 2017 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Mathew S, Chen X, Pozzi A, Zent R. Integrins in renal development. Pediatric nephrology. 2012;27(6):891–900. - PubMed
-
- Pozzi A, Zent R. Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Exp Nephrol. 2003;94(3):e77–84. - PubMed
-
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

