Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study
- PMID: 28043905
- PMCID: PMC5367948
- DOI: 10.1053/j.gastro.2016.12.016
Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study
Abstract
Background & aims: We performed a genome-wide association study (GWAS) to identify genetic risk factors for drug-induced liver injury (DILI) from licensed drugs without previously reported genetic risk factors.
Methods: We performed a GWAS of 862 persons with DILI and 10,588 population-matched controls. The first set of cases was recruited before May 2009 in Europe (n = 137) and the United States (n = 274). The second set of cases were identified from May 2009 through May 2013 from international collaborative studies performed in Europe, the United States, and South America. For the GWAS, we included only cases with patients of European ancestry associated with a particular drug (but not flucloxacillin or amoxicillin-clavulanate). We used DNA samples from all subjects to analyze HLA genes and single nucleotide polymorphisms. After the discovery analysis was concluded, we validated our findings using data from 283 European patients with diagnosis of DILI associated with various drugs.
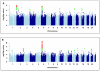
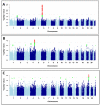
Results: We associated DILI with rs114577328 (a proxy for A*33:01 a HLA class I allele; odds ratio [OR], 2.7; 95% confidence interval [CI], 1.9-3.8; P = 2.4 × 10-8) and with rs72631567 on chromosome 2 (OR, 2.0; 95% CI, 1.6-2.5; P = 9.7 × 10-9). The association with A*33:01 was mediated by large effects for terbinafine-, fenofibrate-, and ticlopidine-related DILI. The variant on chromosome 2 was associated with DILI from a variety of drugs. Further phenotypic analysis indicated that the association between DILI and A*33:01 was significant genome wide for cholestatic and mixed DILI, but not for hepatocellular DILI; the polymorphism on chromosome 2 was associated with cholestatic and mixed DILI as well as hepatocellular DILI. We identified an association between rs28521457 (within the lipopolysaccharide-responsive vesicle trafficking, beach and anchor containing gene) and only hepatocellular DILI (OR, 2.1; 95% CI, 1.6-2.7; P = 4.8 × 10-9). We did not associate any specific drug classes with genetic polymorphisms, except for statin-associated DILI, which was associated with rs116561224 on chromosome 18 (OR, 5.4; 95% CI, 3.0-9.5; P = 7.1 × 10-9). We validated the association between A*33:01 terbinafine- and sertraline-induced DILI. We could not validate the association between DILI and rs72631567, rs28521457, or rs116561224.
Conclusions: In a GWAS of persons of European descent with DILI, we associated HLA-A*33:01 with DILI due to terbinafine and possibly fenofibrate and ticlopidine. We identified polymorphisms that appear to be associated with DILI from statins, as well as 2 non-drug-specific risk factors.
Keywords: Anti-Fungal Agent; Liver Damage; Medication; Side Effect.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Stevens JL, Baker TK. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov Today. 2009;14:162–7. - PubMed
-
- Bjornsson ES, Gunnarsson BI, Grondal G, et al. Risk of drug-induced liver injury from tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2015;13:602–8. - PubMed
-
- Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. 1425, e1–3. quiz e19-20. - PubMed
MeSH terms
Substances
Grants and funding
- U01 DK065211/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- U01 DK083027/DK/NIDDK NIH HHS/United States
- U01 DK083020/DK/NIDDK NIH HHS/United States
- U01 DK065238/DK/NIDDK NIH HHS/United States
- U01 DK065193/DK/NIDDK NIH HHS/United States
- II-LB-0313-20008/DH_/Department of Health/United Kingdom
- U01 DK065176/DK/NIDDK NIH HHS/United States
- U01 DK083023/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 DK082992/DK/NIDDK NIH HHS/United States
- UL1 TR001111/TR/NCATS NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 DK065201/DK/NIDDK NIH HHS/United States
- U01 DK100928/DK/NIDDK NIH HHS/United States
- MR/N005953/1/MRC_/Medical Research Council/United Kingdom
- UL1 TR001108/TR/NCATS NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 DK065184/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

