Donor CYP3A5 genotype influences tacrolimus disposition on the first day after paediatric liver transplantation
- PMID: 28044353
- PMCID: PMC5427244
- DOI: 10.1111/bcp.13219
Donor CYP3A5 genotype influences tacrolimus disposition on the first day after paediatric liver transplantation
Abstract
Aim: The aim of the present study was to investigate the influence of the cytochrome P450 (CYP) 3A4/5 genotype in paediatric liver transplant recipients and donors, and the contribution of age and gender to tacrolimus disposition on the first day after transplantation.
Methods: The contribution of the CYP3A4/5 genotype in paediatric liver transplant recipients and donors to the tacrolimus blood trough concentrations (C0 ) and the tacrolimus concentration/weight-adjusted dose ratio on day 1 was evaluated in 67 liver-transplanted children: 33 boys and 34 girls, mean age 4.5 years.
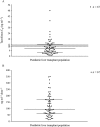
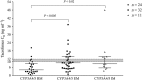
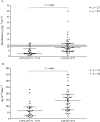
Results: Donor CYP3A5 genotype appears to be significantly associated with tacrolimus disposition on the first day after liver transplantation (P < 0.0002). Other physiological factors, such as recipient age and donor gender may also play a role and lead to significant differences in tacrolimus C0 and tacrolimus concentration/weight-adjusted dose ratio on day 1. However, according to the general linear model, only recipient age appears to be independently associated with tacrolimus disposition on the first day after liver transplantation (P < 0.03). Indeed, there was a faster tacrolimus metabolism in children under 6 years of age (P < 0.02).
Conclusions: Donor CYP3A5 genotype, recipient age and, to a lesser extent, donor gender appear to be associated with tacrolimus disposition on day 1 after transplant. This suggests that increasing the starting tacrolimus doses in paediatric patients under 6 years of age who receive a graft from a male extensive metabolizer may enhance the possibility of their tacrolimus levels reaching the therapeutic range sooner.
Keywords: CYP3A genotyping; children; immunosuppression; liver transplantation; tacrolimus; therapeutic drug monitoring.
© 2017 The British Pharmacological Society.
Figures

References
-
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004; 43: 623–653. - PubMed
-
- Reyes J, Jain A, Mazariegos G, Kashyap R, Green M, Iurlano K, et al. Long‐term results after conversion from cyclosporine to tacrolimus in pediatric liver transplantation for acute and chronic rejection. Transplantation 2000; 69: 2573–2580. - PubMed
-
- Venkataramanan R, Shaw LM, Sarkozi L, Mullins R, Pirsch J, MacFarlane G, et al. Clinical utility of monitoring tacrolimus blood concentrations in liver transplant patients. J Clin Pharmacol 2001; 41: 542–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

