Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity
- PMID: 28045021
- PMCID: PMC5216056
- DOI: 10.1038/ncomms13958
Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity
Abstract
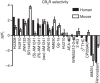
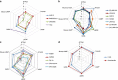
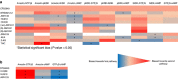
The cannabinoid CB2 receptor (CB2R) represents a promising therapeutic target for various forms of tissue injury and inflammatory diseases. Although numerous compounds have been developed and widely used to target CB2R, their selectivity, molecular mode of action and pharmacokinetic properties have been poorly characterized. Here we report the most extensive characterization of the molecular pharmacology of the most widely used CB2R ligands to date. In a collaborative effort between multiple academic and industry laboratories, we identify marked differences in the ability of certain agonists to activate distinct signalling pathways and to cause off-target effects. We reach a consensus that HU910, HU308 and JWH133 are the recommended selective CB2R agonists to study the role of CB2R in biological and disease processes. We believe that our unique approach would be highly suitable for the characterization of other therapeutic targets in drug discovery research.
Conflict of interest statement
Industry authors U.G., J.F., C.U., B.R. and C.P. are full-time employees of Hoffmann-La Roche and P.P. is full time employee of NIH. All academic authors state that they have no conflict of interest.
Figures


References
-
- Gashaw I., Ellinghaus P., Sommer A. & Asadullah K. What makes a good drug target? Drug Discov. Today 16, 1037–1043 (2011). - PubMed
-
- Van Der Stelt M. et al.. Discovery and optimization of 1-(4-(Pyridin-2-yl)benzyl)imidazolidine-2,4- dione derivatives as a novel class of selective cannabinoid CB2 receptor agonists. J. Med. Chem. 54, 7350–7362 (2011). - PubMed
-
- Kusakabe K. et al.. Selective CB2 agonists with anti-pruritic activity: discovery of potent and orally available bicyclic 2-pyridones. Bioorg. Med. Chem. 21, 3154–3163 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

