Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials
- PMID: 28045115
- PMCID: PMC5332552
- DOI: 10.1038/pcan.2016.62
Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: data from PREVAIL and AFFIRM trials
Abstract
Background: We investigated the impact of skeletal-related events (SREs) on health-related quality of life (HRQoL) in patients with metastatic castration-resistant prostate cancer (mCRPC) in phase III trials of enzalutamide versus placebo.
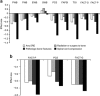
Methods: Patients with mCRPC experiencing at least one SRE during AFFIRM and PREVAIL were assessed for trajectory-adjusted mean change in HRQoL by first SRE using Functional Assessment of Cancer Therapy-Prostate (FACT-P; AFFIRM, three domains, and PREVAIL, nine domains) and EQ-5D (PREVAIL) instruments.
Results: First SREs caused HRQoL deterioration in both trials. Spinal cord compression had the largest impact, with clinically meaningful reductions in seven of nine FACT-P domains in PREVAIL and all three in AFFIRM (mean (95% confidence interval (CI)) change in FACT-P total score -16.95 (-26.47, -7.44) and -9.69 (-16.10, -3.27), respectively). In PREVAIL, first SREs caused clinically meaningful declines in EQ-5D utility index, irrespective of category; spinal cord compression had the largest impact (mean (95% CI) change -0.24 (-0.39, -0.08)). In AFFIRM, FACT-P and FACT-General total scores showed clinically meaningful declines after radiation/surgery to bone.
Conclusions: SREs were associated with clinically meaningful functional declines in the daily lives of patients with mCRPC. Spinal cord compression had the largest impact on HRQoL.
Conflict of interest statement
FS received grants for research, honoraria (speaker and consultant) and serves on the advisory boards of Astellas, Medivation and Janssen. DP and SH and are employees of Astellas Pharma. SA is an employee of Medivation. TMB received grants for research funding from Astellas Pharma, Janssen Research and Development and Medivation, personal (consultancy) fees from Astellas Pharma and Janssen Japan and payment from Research to Practice for participation in a Certified Nursing Education program, which was supported, in part, by Medivation and Astellas Pharma. BT received grants, personal fees and nonfinancial support from Astellas and personal fees from Medivation. YL received personal fees from Astellas (advisory board, speaker, honoraria and travel) and from Medivation (advisory board, speaker). CI is an employee of Quintiles, which received funding from Astellas to conduct the analyses reported here.
Figures

References
-
- Butoescu V, Tombal B. Practical guide to bone health in the spectrum of advanced prostate cancer. Can J Urol 2014; 21 (Suppl 1): 84–92. - PubMed
-
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L et alZoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004; 96: 879–882. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

