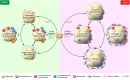
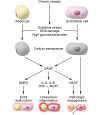
The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis
- PMID: 28045400
- PMCID: PMC5199684
- DOI: 10.1172/JCI88883
The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis
Abstract
There are three dominant contributors to the pathogenesis of dysfunctional adipose tissue (AT) in obesity: unresolved inflammation, inappropriate extracellular matrix (ECM) remodeling and insufficient angiogenic potential. The interactions of these processes during AT expansion reflect both a linear progression as well as feed-forward mechanisms. For example, both inflammation and inadequate angiogenic remodeling can drive fibrosis, which can in turn promote migration of immune cells into adipose depots and impede further angiogenesis. Therefore, the relationship between the members of this triad is complex but important for our understanding of the pathogenesis of obesity. Here we untangle some of these intricacies to highlight the contributions of inflammation, angiogenesis, and the ECM to both "healthy" and "unhealthy" AT expansion.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures