Inhaled nitric oxide for respiratory failure in preterm infants
- PMID: 28045472
- PMCID: PMC6464861
- DOI: 10.1002/14651858.CD000509.pub5
Inhaled nitric oxide for respiratory failure in preterm infants
Abstract
Background: Inhaled nitric oxide (iNO) is effective in term infants with hypoxic respiratory failure. The pathophysiology of respiratory failure and the potential risks of iNO differ substantially in preterm infants, necessitating specific study in this population.
Objectives: To determine effects of treatment with inhaled nitric oxide (iNO) on death, bronchopulmonary dysplasia (BPD), intraventricular haemorrhage (IVH) or other serious brain injury and on adverse long-term neurodevelopmental outcomes in preterm newborn infants with hypoxic respiratory failure.Owing to substantial variation in study eligibility criteria, which decreases the utility of an overall analysis, we divided participants post hoc into three groups: (1) infants treated over the first three days of life because of defects in oxygenation, (2) preterm infants with evidence of pulmonary disease treated routinely with iNO and (3) infants treated later (after three days of age) because of elevated risk of BPD.
Search methods: We used standard methods of the Cochrane Neonatal Review Group. We searched MEDLINE, Embase, Healthstar and the Cochrane Central Register of Controlled Trials in the Cochrane Library through January 2016. We also searched the abstracts of the Pediatric Academic Societies.
Selection criteria: Eligible for inclusion were randomised and quasi-randomised studies in preterm infants with respiratory disease that compared effects of iNO gas versus control, with or without placebo.
Data collection and analysis: We used standard methods of the Cochrane Neonatal Review Group and applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
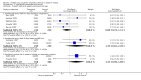
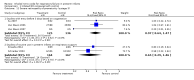
Main results: We found 17 randomised controlled trials of iNO therapy in preterm infants. We grouped these trials post hoc into three categories on the basis of entry criteria: treatment during the first three days of life for impaired oxygenation, routine use in preterm babies along with respiratory support and later treatment for infants at increased risk for bronchopulmonary dysplasia (BPD). We performed no overall analyses.Eight trials providing early rescue treatment for infants on the basis of oxygenation criteria demonstrated no significant effect of iNO on mortality or BPD (typical risk ratio (RR) 0.94, 95% confidence interval (CI) 0.87 to 1.01; 958 infants). Four studies examining routine use of iNO in infants with pulmonary disease reported no significant reduction in death or BPD (typical RR 0.94, 95% CI 0.87 to 1.02; 1924 infants), although this small effect approached significance. Later treatment with iNO based on risk of BPD (three trials) revealed no significant benefit for this outcome in analyses of summary data (typical RR 0.92, 95% CI 0.85 to 1.01; 1075 infants).Investigators found no clear effect of iNO on the frequency of all grades of IVH nor severe IVH. Early rescue treatment was associated with a non-significant 20% increase in severe IVH.We found no effect on the incidence of neurodevelopmental impairment.
Authors' conclusions: iNO does not appear to be effective as rescue therapy for the very ill preterm infant. Early routine use of iNO in preterm infants with respiratory disease does not prevent serious brain injury or improve survival without BPD. Later use of iNO to prevent BPD could be effective, but current 95% confidence intervals include no effect; the effect size is likely small (RR 0.92) and requires further study.
Conflict of interest statement
Dr Barrington was Chair of the Canadian Medical Advisory Committee for iNO therapeutics for one meeting in 2004. Dr Barrington and Dr Finer were participants in a research project funded by Ikaria, an individual patient data meta‐analysis of iNO in the preterm, from which neither received any income.
Dr Barrington received an honorarium for speaking at a symposium funded by Mallinckrodt on the topic of probiotics.
Figures












Update of
-
Inhaled nitric oxide for respiratory failure in preterm infants.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD000509. doi: 10.1002/14651858.CD000509.pub4. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2017 Jan 03;1:CD000509. doi: 10.1002/14651858.CD000509.pub5. PMID: 21154346 Updated.
References
References to studies included in this review
Ballard 2006 {published data only}
-
- Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. New England Jounal of Medicine 2006;355:343‐53. - PubMed
-
- Fiore JM, Hibbs AM, Zadell AE, Merrill JD, Eichenwald EC, Puri AR, et al. The effect of inhaled nitric oxide on pulmonary function in preterm infants. Journal of Perinatology 2007;27:766‐71. - PubMed
Dani 2006 {published data only}
-
- Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF. Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome. Acta Paediatrica 2006;95:1116‐23. - PubMed
EUNO 2009 {published data only}
-
- Durrmeyer X, Hummler H, Sanchez‐Luna M, Carnielli VP, Field D, Greenough A, et al. Two‐year outcomes of a randomized controlled trial of inhaled nitric oxide in premature infants. Pediatrics 2013;132(3):e695‐703. [PUBMED: 23940237] - PubMed
-
- Mercier JC, Hummler H, Durrmeyer X, Sanchez‐Luna M, Carnielli V, Field D, et al. Inhaled nitric oxide for the prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomized controlled trial. Lancet 2010;376:346‐54. - PubMed
Hascoet 2005 {published and unpublished data}
-
- Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A, et al. The safety and efficacy of nitric oxide therapy in premature infants. Journal of Pediatrics 2005;146:318‐23. - PubMed
INNOVO 2005 {published data only}
-
- Ahluwalia J, Tooley J, Cheema I, Sweet DG, Curley AE, Halliday HL, et al. A dose response study of inhaled nitric oxide in hypoxic respiratory failure in preterm infants. Early Human Development 2006;82:477‐83. - PubMed
-
- Field D, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC, et al. Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomized controlled trial (ISRCTN 17821339). Pediatrics 2005;115:926‐36. - PubMed
-
- Huddy CL, Bennett CC, Hardy P, Field D, Elbourne D, Grieve R, et al. The INNOVO multicentre randomised controlled trial: neonatal ventilation with inhaled nitric oxide versus ventilatory support without nitric oxide for severe respiratory failure in preterm infants: follow up at 4‐5 years. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93:F430‐5. - PubMed
Kinsella 1999 {published data only}
-
- Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomized controlled trial. Lancet 1999;354:1061‐5. - PubMed
Kinsella 2006 {published and unpublished data}
-
- Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. New England Journal of Medicine 2006;355:354‐64. - PubMed
-
- Watson RS, Clermont G, Kinsella JP, Kong L, Arendt RE, Cutter G, et al. Clinical and economic effects of iNO in premature newborns with respiratory failure at 1 year. Pediatrics 2009;124:1333‐43. - PubMed
Kinsella 2014 {published data only}
Mercier 1999 {published data only}
-
- Franco‐Belgium Collaborative NO Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomized controlled trial. Lancet 1999;354:1066‐71. - PubMed
-
- Mercier JC, Dehan M, Breart G, Clement S, O'Nody P. Inhaled nitric oxide in neonatal respiratory failure. A randomized clinical trial. Pediatric Research 1998;43:290A (Abstract).
-
- Truffert P, Llado‐Paris J, Mercier JC, Dehan M, Bréart G, Franco‐Belgian iNO Study Group. Early inhaled nitric oxide in moderately hypoxemic preterm and term newborns with RDS: the RDS subgroup analysis of the Franco‐Belgian iNO Randomized Trial. European Journal of Pediatrics 2003;162:646‐7. - PubMed
Schreiber 2003 {published data only}
-
- Mestan K, Marks J, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. New England Journal of Medicine 2005;353:23‐32. - PubMed
-
- Schreiber MD, Gin‐Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. New England Journal of Medicine 2003;349:2099‐107. - PubMed
Srisuparp 2002 {published data only}
-
- Srisuparp P, Heitschmidt M, Schreiber MD. Inhaled nitric oxide therapy in premature infants with mild to moderate respiratory distress syndrome. Journal of the Medical Association of Thailand 2002;85:S469‐78. - PubMed
Su 2007 {published data only}
-
- Su PH, Chen JY. Inhaled nitric oxide in the management of preterm infants with severe respiratory failure. Journal of Perinatology 2008;28:112‐6. - PubMed
Subhedar 1997 {published data only}
-
- Bennett AJ, Shaw NJ, Gregg JE, Subhedar NV. Neurodevelopmental outcome in high‐risk preterm infants treated with inhaled nitric oxide. Acta Paediatrica 2001;90:573‐6. - PubMed
Van Meurs 2005 {published data only}
-
- Meurs KP, Wright L, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. New England Journal of Medicine 2005;353:13‐22. - PubMed
Van Meurs 2007 {published data only}
-
- Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide in infants >1500 g and <34 weeks gestation with severe respiratory failure. Journal of Perinatology 2007;27:347‐52. - PubMed
Wei 2014 {published data only}
-
- Wei QF, Pan XN, Li Y, Feng L, Yao LP, Liu GL, et al. Efficacy of inhaled nitric oxide in premature infants with hypoxic respiratory failure. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(8):805‐9. [PUBMED: 25140772] - PubMed
Yoder 2013 {published data only}
-
- Yoder BA. Inhaled NO for prevention of BPD: update on the NEWNO Trial. Hot Topics in Neonatology; 2013 December 8; Washington, DC. 2013.
References to studies excluded from this review
Day 1996 {published data only}
-
- Day RW, Lynch JM, White KS, Ward RM. Acute response to inhaled nitric oxide in newborns with respiratory failure and pulmonary hypertension. Pediatrics 1996;98:698‐705. - PubMed
Lindwall 2005 {published data only}
-
- Lindwall R, Blennow M, Svensson M, Jonsson B, Berggren‐Bostrom E, Flanby M, et al. A pilot study of inhaled nitric oxide in preterm infants treated with nasal continuous positive airway pressure for respiratory distress syndrome. Intensive Care Medicine 2005;31:959‐64. - PubMed
Skimming 1997 {published data only}
-
- Skimming JW, Bender KA, Hutchison AA, Drummond WH. Nitric oxide inhalation in infants with respiratory distress syndrome. Journal of Pediatrics 1997;130:225‐30. - PubMed
Additional references
Abman 1990
-
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium‐derived relaxing factor during transition of pulmonary circulation at birth. American Journal of Physiology 1990;259:H1921‐7. - PubMed
Abman 1993
-
- Abman SH, Kinsella JP, Schaffer MS, Wilkening RB. Inhaled nitric oxide in the management of a premature newborn with severe respiratory distress and pulmonary hypertension. Pediatrics 1993;92:606‐9. - PubMed
Askie 2010
Bland 2005
Cornfield 1992
-
- Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH. Effects of birth‐related stimuli on L‐arginine‐dependent pulmonary vasodilation in ovine fetus. American Journal of Physiology 1992;262:H1474‐81. - PubMed
Finer 1998
-
- Finer NN, Barrington KJ. Nitric oxide therapy for the newborn infant. Seminars in Neonatology 1998;3:127‐36. - PubMed
Finer 2000
Frostell 1991
-
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991;83:2038‐47. - PubMed
GRADEpro [Computer program]
-
- McMaster University, 2014. GRADEpro [www.gradepro.org]. Ontario: McMaster University, 2014.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hoehn 2006
-
- Hoehn T, Krause MF, Buhrer C. Meta‐analysis of inhaled nitric oxide in premature infants: an update. Klinische Padiatrie 2006;218:57‐61. - PubMed
Hogman 1993
-
- Hogman M, Frostell C, Arnberg H, Hedenstierna G. Bleeding time prolongation and NO inhalation. Lancet 1993;341:1664‐5. - PubMed
Kinsella 1992
-
- Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. American Journal of Physiology 1992;263:H875‐80. - PubMed
Kinsella 1994
-
- Kinsella JP, Ivy DD, Abman SH. Inhaled nitric oxide improves gas exchange and lowers pulmonary vascular resistance in severe experimental hyaline membrane disease. Pediatric Research 1994;36:402‐8. - PubMed
McAndrew 1997
-
- McAndrew J, Patel RP, Jo H, Cornwell T, Lincoln T, Moellering D, et al. The interplay of nitric oxide and peroxynitrite with signal transduction pathways: implications for disease. Seminars in Perinatology 1997;21:351‐66. - PubMed
McCurnin 2005
-
- McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne‐Lawrence S, Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. American Journal of Physiology. Lung Cellular and Molecular Physiology 2005;288:L450‐9. - PMC - PubMed
Peliowski 1995
-
- Peliowski A, Finer N, Etches P, Tierney AJ, Ryan CA. Inhaled nitric oxide for premature infants after prolonged rupture of the membranes. Journal of Pediatrics 1995;126:450‐3. - PubMed
Roberts 1993
-
- Roberts JD Jr, Chen TY, Kawai N, Wain J, Dupuy P, Shimouchi A, et al. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circulation Research 1993;72:246‐54. - PubMed
Rossaint 1993
-
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. New England Journal of Medicine 1993;328:399‐405. - PubMed
Ryan 1996
-
- Ryan SW, Nycyk J, Shaw BNJ. Prediction of chronic neonatal lung disease on day 4 of life. European Journal of Pediatrics 1996;155:668‐71. - PubMed
Samama 1995
-
- Samama CM, Diaby M, Fellahi JL Mdhafar A, Eyraud D, Arock M. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 1995;83:56‐65. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated October 2013.
Skimming 1995
-
- Skimming JW, DeMarco VG, Cassin S. The effects of nitric oxide inhalation on the pulmonary circulation of preterm lambs. Pediatric Research 1995;37:35‐40. - PubMed
Soll 2001
Van Meurs 1997
-
- Meurs KP, Rhine WD, Asselin JM, Durand DJ, Premie INO Collaborative Group. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Pediatric Research 1997;41:271A (Abstract). - PubMed
Vohr 2005
-
- Vohr BR, Wright LL, Poole K, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics 2005;116:635‐43. - PubMed
Walther 1992
-
- Walther FJ, Benders MJ, Leighton JO. Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics 1992;90:899‐904. - PubMed
Wirbelauer 2010
-
- Wirbelauer J, Speer CP. Significance of nitric oxide inhalation (NO) in preterm infants < 34 weeks of gestation [Stellenwert der Inhalation von Stickstoffmonoxid (NO) bei Fruhgeborenen<34 Gestationswochen.]. Klinische Padiatrie 2010;222:56‐61. - PubMed
Wood 2005
-
- Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90:F134‐40. - PMC - PubMed
References to other published versions of this review
Barrington 1999
-
- Barrington KJ, Finer NN. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000509] - DOI
Barrington 2001
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

