Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance)
- PMID: 28045625
- PMCID: PMC5455353
- DOI: 10.1200/JCO.2016.69.4406
Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance)
Abstract
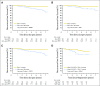
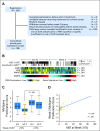
Purpose To determine the pathologic complete response (pCR) rate in estrogen receptor (ER) -positive primary breast cancer triaged to chemotherapy when the protein encoded by the MKI67 gene (Ki67) level was > 10% after 2 to 4 weeks of neoadjuvant aromatase inhibitor (AI) therapy. A second objective was to examine risk of relapse using the Ki67-based Preoperative Endocrine Prognostic Index (PEPI). Methods The American College of Surgeons Oncology Group (ACOSOG) Z1031A trial enrolled postmenopausal women with stage II or III ER-positive (Allred score, 6 to 8) breast cancer whose treatment was randomly assigned to neoadjuvant AI therapy with anastrozole, exemestane, or letrozole. For the trial ACOSOG Z1031B, the protocol was amended to include a tumor Ki67 determination after 2 to 4 weeks of AI. If the Ki67 was > 10%, patients were switched to neoadjuvant chemotherapy. A pCR rate of > 20% was the predefined efficacy threshold. In patients who completed neoadjuvant AI, stratified Cox modeling was used to assess whether time to recurrence differed by PEPI = 0 score (T1 or T2, N0, Ki67 < 2.7%, ER Allred > 2) versus PEPI > 0 disease. Results Only two of the 35 patients in ACOSOG Z1031B who were switched to neoadjuvant chemotherapy experienced a pCR (5.7%; 95% CI, 0.7% to 19.1%). After 5.5 years of median follow-up, four (3.7%) of the 109 patients with a PEPI = 0 score relapsed versus 49 (14.4%) of 341 of patients with PEPI > 0 (recurrence hazard ratio [PEPI = 0 v PEPI > 0], 0.27; P = .014; 95% CI, 0.092 to 0.764). Conclusion Chemotherapy efficacy was lower than expected in ER-positive tumors exhibiting AI-resistant proliferation. The optimal therapy for these patients should be further investigated. For patients with PEPI = 0 disease, the relapse risk over 5 years was only 3.6% without chemotherapy, supporting the study of adjuvant endocrine monotherapy in this group. These Ki67 and PEPI triage approaches are being definitively studied in the ALTERNATE trial (Alternate Approaches for Clinical Stage II or III Estrogen Receptor Positive Breast Cancer Neoadjuvant Treatment in Postmenopausal Women: A Phase III Study; clinical trial information: NCT01953588).
Figures



Comment in
-
Put Some PEPI in Your Step: Ki67's Long Road to Respectability.J Clin Oncol. 2017 Apr 1;35(10):1031-1032. doi: 10.1200/JCO.2016.71.2182. Epub 2017 Feb 21. J Clin Oncol. 2017. PMID: 28221864 No abstract available.
References
-
- Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 - PubMed
-
- Eiermann W, Paepke S, Appfelstaedt J, et al. : Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12:1527-1532, 2001 - PubMed
-
- Ellis MJ, Coop A, Singh B, et al. : Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol 19:3808-3816, 2001 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA047577/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- U01 CA114722/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- U10 CA180857/CA/NCI NIH HHS/United States
- U10 CA105409/CA/NCI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- U10 CA076001/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA180870/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U24 CA114736/CA/NCI NIH HHS/United States
- R01 CA095614/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

