Human Leukocytes Kill Brugia malayi Microfilariae Independently of DNA-Based Extracellular Trap Release
- PMID: 28045905
- PMCID: PMC5234842
- DOI: 10.1371/journal.pntd.0005279
Human Leukocytes Kill Brugia malayi Microfilariae Independently of DNA-Based Extracellular Trap Release
Abstract
Background: Wuchereria bancrofti, Brugia malayi and Brugia timori infect over 100 million people worldwide and are the causative agents of lymphatic filariasis. Some parasite carriers are amicrofilaremic whilst others facilitate mosquito-based disease transmission through blood-circulating microfilariae (Mf). Recent findings, obtained largely from animal model systems, suggest that polymorphonuclear leukocytes (PMNs) contribute to parasitic nematode-directed type 2 immune responses. When exposed to certain pathogens PMNs release extracellular traps (NETs) in the form of chromatin loaded with various antimicrobial molecules and proteases.
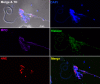
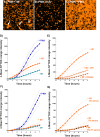
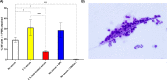
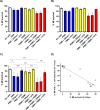
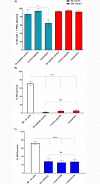
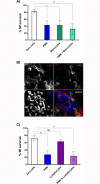
Principal findings: In vitro, PMNs expel large amounts of NETs that capture but do not kill B. malayi Mf. NET morphology was confirmed by fluorescence imaging of worm-NET aggregates labelled with DAPI and antibodies to human neutrophil elastase, myeloperoxidase and citrullinated histone H4. A fluorescent, extracellular DNA release assay was used to quantify and observe Mf induced NETosis over time. Blinded video analyses of PMN-to-worm attachment and worm survival during Mf-leukocyte co-culture demonstrated that DNase treatment eliminates PMN attachment in the absence of serum, autologous serum bolsters both PMN attachment and PMN plus peripheral blood mononuclear cell (PBMC) mediated Mf killing, and serum heat inactivation inhibits both PMN attachment and Mf killing. Despite the effects of heat inactivation, the complement inhibitor compstatin did not impede Mf killing and had little effect on PMN attachment. Both human PMNs and monocytes, but not lymphocytes, are able to kill B. malayi Mf in vitro and NETosis does not significantly contribute to this killing. Leukocytes derived from presumably parasite-naïve U.S. resident donors vary in their ability to kill Mf in vitro, which may reflect the pathological heterogeneity associated with filarial parasitic infections.
Conclusions/significance: Human innate immune cells are able to recognize, attach to and kill B. malayi microfilariae in an in vitro system. This suggests that, in vivo, the parasites can evade this ability, or that only some human hosts support an infection with circulating Mf.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Recognition and killing of Brugia malayi microfilariae by human immune cells is dependent on the parasite sample and is not altered by ivermectin treatment.Int J Parasitol Drugs Drug Resist. 2018 Dec;8(3):587-595. doi: 10.1016/j.ijpddr.2018.09.002. Epub 2018 Sep 22. Int J Parasitol Drugs Drug Resist. 2018. PMID: 30279092 Free PMC article.
-
Humans from Wuchereria bancrofti endemic area elicit substantial immune response to proteins of the filarial parasite Brugia malayi and its endosymbiont Wolbachia.Parasit Vectors. 2017 Jan 24;10(1):40. doi: 10.1186/s13071-016-1963-x. Parasit Vectors. 2017. PMID: 28118850 Free PMC article.
-
Species-specific and cross-reactive antigen of filarial worm: Brugia malayi and Setaria digitata.J Vector Borne Dis. 2019 Oct-Dec;56(4):323-329. doi: 10.4103/0972-9062.302035. J Vector Borne Dis. 2019. PMID: 33269732
-
Recent advances in the application of molecular biology in filariasis.Southeast Asian J Trop Med Public Health. 1993;24 Suppl 2:55-63. Southeast Asian J Trop Med Public Health. 1993. PMID: 7973949 Review.
-
Examining the role of macrolides and host immunity in combatting filarial parasites.Parasit Vectors. 2017 Apr 14;10(1):182. doi: 10.1186/s13071-017-2116-6. Parasit Vectors. 2017. PMID: 28410595 Free PMC article. Review.
Cited by
-
Characterization of Two Trichinella spiralis Adult-Specific DNase II and Their Capacity to Induce Protective Immunity.Front Microbiol. 2018 Nov 5;9:2504. doi: 10.3389/fmicb.2018.02504. eCollection 2018. Front Microbiol. 2018. PMID: 30455671 Free PMC article.
-
NETosis in Parasitic Infections: A Puzzle That Remains Unsolved.Int J Mol Sci. 2023 May 19;24(10):8975. doi: 10.3390/ijms24108975. Int J Mol Sci. 2023. PMID: 37240321 Free PMC article. Review.
-
Canine Angiostrongylus vasorum-Induced Early Innate Immune Reactions Based on NETs Formation and Canine Vascular Endothelial Cell Activation In Vitro.Biology (Basel). 2021 May 12;10(5):427. doi: 10.3390/biology10050427. Biology (Basel). 2021. PMID: 34065858 Free PMC article.
-
Antibody responses against the vaccine antigens Ov-103 and Ov-RAL-2 are associated with protective immunity to Onchocerca volvulus infection in both mice and humans.PLoS Negl Trop Dis. 2019 Sep 16;13(9):e0007730. doi: 10.1371/journal.pntd.0007730. eCollection 2019 Sep. PLoS Negl Trop Dis. 2019. PMID: 31525197 Free PMC article.
-
Anthelmintics - From Discovery to Resistance III (Indian Rocks Beach, FL, 2018).Int J Parasitol Drugs Drug Resist. 2018 Dec;8(3):494-495. doi: 10.1016/j.ijpddr.2018.11.002. Epub 2018 Nov 5. Int J Parasitol Drugs Drug Resist. 2018. PMID: 30429103 Free PMC article.
References
-
- McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: Defining mechanisms and mediators. Int J Parasit. 2013;43: 301–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

