An Experimental Analysis of the Molecular Effects of Trastuzumab (Herceptin) and Fulvestrant (Falsodex), as Single Agents or in Combination, on Human HR+/HER2+ Breast Cancer Cell Lines and Mouse Tumor Xenografts
- PMID: 28045951
- PMCID: PMC5207527
- DOI: 10.1371/journal.pone.0168960
An Experimental Analysis of the Molecular Effects of Trastuzumab (Herceptin) and Fulvestrant (Falsodex), as Single Agents or in Combination, on Human HR+/HER2+ Breast Cancer Cell Lines and Mouse Tumor Xenografts
Erratum in
-
Correction: An Experimental Analysis of the Molecular Effects of Trastuzumab (Herceptin) and Fulvestrant (Falsodex), as Single Agents or in Combination, on Human HR+/HER2+ Breast Cancer Cell Lines and Mouse Tumor Xenografts.PLoS One. 2024 Sep 23;19(9):e0311128. doi: 10.1371/journal.pone.0311128. eCollection 2024. PLoS One. 2024. PMID: 39312556 Free PMC article.
Abstract
Purpose: To investigate the effects of trastuzumab (herceptin) and fulvestrant (falsodex) either in combination or alone, on downstream cell signaling pathways in lab-cultured human HR+/HER2+ breast cancer cell lines ZR-75-1 and BT-474, as well as on protein expression levels in mouse xenograft tissue.
Methods: Cells were cultivated in the presence of trastuzumab or fulvestrant or both. Molecular events that resulted in an inhibition of cell proliferation and cell cycle progression or in an increased rate of apoptosis were studied. The distribution and abundance of the proteins p-Akt and p-Erk expressed in these cells in response to single agents or combinatorial treatment were also investigated. In addition, the effects of trastuzumab and fulvestrant, either as single agents or in combination on tumor growth as well as on expression of the protein p-MED1 expressed in in vivo mouse xenograft models was also examined.
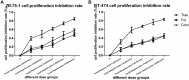
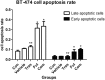
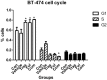
Results: Cell proliferation was increasingly inhibited by trastuzumab or fulvestrant or both, with a CI<1 and DRI>1 in both human cell lines. The rate of apoptosis increased only in the BT-474 cell line and not in the ZR-75-1 cell line upon treatment with fulvestrant and not trastuzumab as a single agent (P<0.05). Interestingly, fulvestrant, in combination with trastuzumab, did not significantly alter the rate of apoptosis (in comparison with fulvestrant alone), in the BT-474 cell line (P>0.05). Cell accumulation in the G1 phase of cell cycle was investigated in all treatment groups (P<0.05), and the combination of trastuzumab and fulvestrant reversed the effects of fulvestrant alone on p-Akt and p-Erk protein expression levels. Using ZR-75-1 or BT-474 to generate in vivo tumor xenografts in BALB/c athymic mouse models, we showed that a combination of both drugs resulted in a stronger inhibition of tumor growth (P<0.05) and a greater decrease in the levels of activated MED1 (p-MED1) expressed in tumor issues compared with the use of either drug as a single agent.
Conclusions: We demonstrate that the administration of trastuzumab and fulvestrant in combination results in positive synergistic effects on both, ZR-75-1 and BT-474 cell lines. This combinatorial approach is likely to reduce physiological side effects of both drugs, thus providing a theoretical basis for the use of such combination treatment in order to resolve HR+/HER2+ triple positive breast cancer that has previously been shown to be resistant to endocrine treatment alone.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

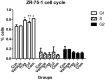
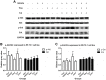
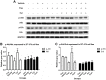
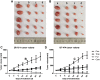


References
-
- Parra-Palau JL, Pedersen K, Peg V, Scaltriti M, Angelini PD, Escorihuela M, et al. A major role of p95/611-CTF, a carboxy-terminal fragment of HER2, in the down-modulation of the estrogen receptor in HER2-positive breast cancers. Cancer Res 2010. November 1;70(21):8537–46. 10.1158/0008-5472.CAN-10-1701 - DOI - PubMed
-
- Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015. July;16(7):816–29. 10.1016/S1470-2045(15)00051-0 - DOI - PubMed
-
- Krop IE, Suter TM, Dang CT, Dirix L, Romieu G, Zamagni C, et al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol 2015. April 1;33(10):1136–42. 10.1200/JCO.2014.58.7782 - DOI - PMC - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987. January 9;235(4785):177–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

