The Role of Aneuploidy in Cancer Evolution
- PMID: 28049655
- PMCID: PMC5204330
- DOI: 10.1101/cshperspect.a028373
The Role of Aneuploidy in Cancer Evolution
Abstract
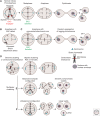
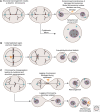
Chromosomal aberrations during cell division represent one of the first recognized features of human cancer cells, and modern detection methods have revealed the pervasiveness of aneuploidy in cancer. The ongoing karyotypic changes brought about by chromosomal instability (CIN) contribute to tumor heterogeneity, drug resistance, and treatment failure. Whole-chromosome and segmental aneuploidies resulting from CIN have been proposed to allow "macroevolutionary" leaps that may contribute to profound phenotypic change. In this review, we will outline evidence indicating that aneuploidy and CIN contribute to cancer evolution.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
