Allosteric Transmission along a Loosely Structured Backbone Allows a Cardiac Troponin C Mutant to Function with Only One Ca2+ Ion
- PMID: 28049727
- PMCID: PMC5313108
- DOI: 10.1074/jbc.M116.765362
Allosteric Transmission along a Loosely Structured Backbone Allows a Cardiac Troponin C Mutant to Function with Only One Ca2+ Ion
Abstract
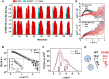
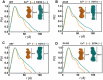
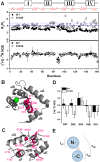
Hypertrophic cardiomyopathy (HCM) is one of the most common cardiomyopathies and a major cause of sudden death in young athletes. The Ca2+ sensor of the sarcomere, cardiac troponin C (cTnC), plays an important role in regulating muscle contraction. Although several cardiomyopathy-causing mutations have been identified in cTnC, the limited information about their structural defects has been mapped to the HCM phenotype. Here, we used high-resolution electron-spray ionization mass spectrometry (ESI-MS), Carr-Purcell-Meiboom-Gill relaxation dispersion (CPMG-RD), and affinity measurements of cTnC for the thin filament in reconstituted papillary muscles to provide evidence of an allosteric mechanism in mutant cTnC that may play a role to the HCM phenotype. We showed that the D145E mutation leads to altered dynamics on a μs-ms time scale and deactivates both of the divalent cation-binding sites of the cTnC C-domain. CPMG-RD captured a low populated protein-folding conformation triggered by the Glu-145 replacement of Asp. Paradoxically, although D145E C-domain was unable to bind Ca2+, these changes along its backbone allowed it to attach more firmly to thin filaments than the wild-type isoform, providing evidence for an allosteric response of the Ca2+-binding site II in the N-domain. Our findings explain how the effects of an HCM mutation in the C-domain reflect up into the N-domain to cause an increase of Ca2+ affinity in site II, thus opening up new insights into the HCM phenotype.
Keywords: calcium-binding protein; cardiomyopathy; nuclear magnetic resonance (NMR); protein structure; small-angle X-ray scattering (SAXS); troponin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

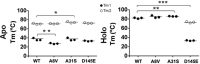


References
-
- Lan F., Lee A. S., Liang P., Sanchez-Freire V., Nguyen P. K., Wang L., Han L., Yen M., Wang Y., Sun N., Abilez O. J, Hu S., Ebert A. D., Navarrete E. G., Simmons C. S., et al. (2013) Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 12, 101–113 - PMC - PubMed
-
- Writing Group Members, Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., Das S. R., de Ferranti S., Després J. P., Fullerton H. J., Howard V. J., Huffman M. D., Isasi C. R., Jiménez M. C., Judd S. E., et al. (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e60 - PubMed
-
- Maron B. J., Towbin J. A., Thiene G., Antzelevitch C., Corrado D., Arnett D., Moss A. J., Seidman C. E., Young J. B., American Heart Association, Council on Clinical Cardiology, Heart Failure and Transplantation Committee, Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups, and Council on Epidemiology and Prevention (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113, 1807–1816 - PubMed
-
- Landstrom A. P., Parvatiyar M. S., Pinto J. R., Marquardt M. L., Bos J. M., Tester D. J., Ommen S. R., Potter J. D., and Ackerman M. J. (2008) Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J. Mol. Cell. Cardiol. 45, 281–288 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

