Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals
- PMID: 28049818
- PMCID: PMC5255593
- DOI: 10.1073/pnas.1606722113
Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals
Abstract
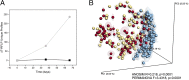
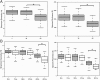
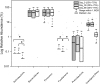
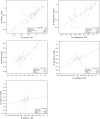
Directed modulation of the colonic bacteria to metabolize lactose effectively is a potentially useful approach to improve lactose digestion and tolerance. A randomized, double-blind, multisite placebo-controlled trial conducted in human subjects demonstrated that administration of a highly purified (>95%) short-chain galactooligosaccharide (GOS), designated "RP-G28," significantly improved clinical outcomes for lactose digestion and tolerance. In these individuals, stool samples were collected pretreatment (day 0), after GOS treatment (day 36), and 30 d after GOS feeding stopped and consumption of dairy products was encouraged (day 66). In this study, changes in the fecal microbiome were investigated using 16S rRNA amplicon pyrosequencing and high-throughput quantitative PCR. At day 36, bifidobacterial populations were increased in 27 of 30 of GOS subjects (90%), demonstrating a bifidogenic response in vivo. Relative abundance of lactose-fermenting Bifidobacterium, Faecalibacterium, and Lactobacillus were significantly increased in response to GOS. When dairy was introduced into the diet, lactose-fermenting Roseburia species increased from day 36 to day 66. The results indicated a definitive change in the fecal microbiome of lactose-intolerant individuals, increasing the abundance of lactose-metabolizing bacteria that were responsive to dietary adaptation to GOS. This change correlated with clinical outcomes of improved lactose tolerance.
Keywords: GOS; lactose intolerance; microbiome; prebiotic.
Conflict of interest statement
This study was funded by Ritter Pharmaceuticals, Inc., of which A.J.R. is the founder, and by the North Carolina Agriculture Foundation. T.R.K. and D.S. are on the scientific advisory board for Ritter Pharmaceuticals, Inc. and assisted in the design, management, and conduct of the study and in the interpretation of data from the study. The work at the University of North Carolina at Chapel Hill Microbiome Center also was funded in part by Ritter Pharmaceuticals, Inc. and by the North Carolina Agriculture Foundation. Ritter Pharmaceuticals, Inc. was the sole source of highly purified galactooligosaccharide (>95%) GOS that was available for the study. The results of these studies have been scientifically judged and interpreted independently by the authors without bias based on the funding or industrial partners involved.
Figures







References
-
- Scrimshaw NS, Murray EB. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr. 1988;48(4) Suppl:1079–1159. - PubMed
-
- Hertzler SR, Savaiano DA, Levitt MD. Fecal hydrogen production and consumption measurements. Response to daily lactose ingestion by lactose maldigesters. Dig Dis Sci. 1997;42(2):348–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

