Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration
- PMID: 28049831
- PMCID: PMC5240690
- DOI: 10.1073/pnas.1616811114
Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration
Abstract
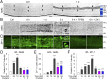
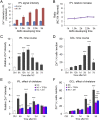
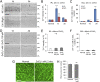
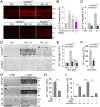
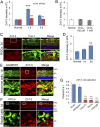
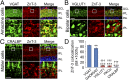
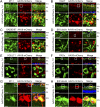
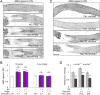
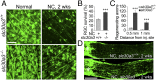
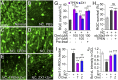
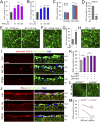
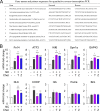
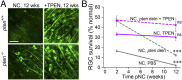
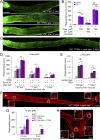
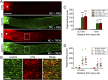
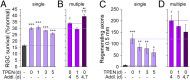
Retinal ganglion cells (RGCs), the projection neurons of the eye, cannot regenerate their axons once the optic nerve has been injured and soon begin to die. Whereas RGC death and regenerative failure are widely viewed as being cell-autonomous or influenced by various types of glia, we report here that the dysregulation of mobile zinc (Zn2+) in retinal interneurons is a primary factor. Within an hour after the optic nerve is injured, Zn2+ increases several-fold in retinal amacrine cell processes and continues to rise over the first day, then transfers slowly to RGCs via vesicular release. Zn2+ accumulation in amacrine cell processes involves the Zn2+ transporter protein ZnT-3, and deletion of slc30a3, the gene encoding ZnT-3, promotes RGC survival and axon regeneration. Intravitreal injection of Zn2+ chelators enables many RGCs to survive for months after nerve injury and regenerate axons, and enhances the prosurvival and regenerative effects of deleting the gene for phosphatase and tensin homolog (pten). Importantly, the therapeutic window for Zn2+ chelation extends for several days after nerve injury. These results show that retinal Zn2+ dysregulation is a major factor limiting the survival and regenerative capacity of injured RGCs, and point to Zn2+ chelation as a strategy to promote long-term RGC protection and enhance axon regeneration.
Keywords: amacrine cell; cell death; chelation; exocytosis; neuroprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

