Treatment patterns and survival in patients with ALK- positive non-small-cell lung cancer: a Canadian retrospective study
- PMID: 28050149
- PMCID: PMC5176386
- DOI: 10.3747/co.23.3273
Treatment patterns and survival in patients with ALK- positive non-small-cell lung cancer: a Canadian retrospective study
Abstract
Background: Crizotinib was the first agent approved for the treatment of anaplastic lymphoma kinase (ALK)-positive (+) non-small-cell lung cancer (nsclc), followed by ceritinib. However, patients eventually progress or develop resistance to crizotinib. With limited real-world data available, the objective of the present work was to evaluate treatment patterns and survival after crizotinib in patients with locally advanced or metastatic ALK+ nsclc in Canada.
Methods: In this retrospective study at 6 oncology centres across Canada, medical records of patients with locally advanced or metastatic ALK+ nsclc were reviewed. Demographic and clinical characteristics, treatments, and outcomes data were abstracted. Analyses focused on patients who discontinued crizotinib treatment.
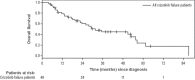
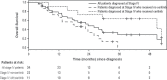
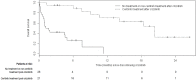
Results: Of the 97 patients included, 9 were crizotinib-naïve, and 39 were still receiving crizotinib at study end. The 49 patients who discontinued crizotinib treatment were included in the analysis. Of those 49 patients, 43% received ceritinib at any time, 20% subsequently received systemic chemotherapy only (but never ceritinib), and 37% received no further treatment or died before receiving additional treatment. Median overall survival from crizotinib discontinuation was shorter in patients who did not receive ceritinib than in those who received ceritinib (1.7 months vs. 20.4 months, p < 0.001). In a multivariable analysis, factors associated with poorer survival included lack of additional therapies (particularly ceritinib), male sex, and younger age, but not smoking status; patients of Asian ethnicity showed a nonsignificant trend toward improved survival.
Conclusions: A substantial proportion of patients with ALK+ nsclc received no further treatment or died before receiving additional treatment after crizotinib. Treatment with systemic agents was associated with improved survival, with ceritinib use being associated with the longest survival.
Keywords: ALK-positive non-small-cell lung cancer; Crizotinib; ceritinib; lung cancer; survival; treatment patterns.
Figures


References
-
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015.
-
- Health Canada . Notice of Compliance–Authorization with Conditions for Xalkori [Web page] Ottawa, ON: Health Canada; 2015. [Available at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/notices-avis/conditions/index-...; cited 16 March 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources

