Evolutionary conservation and in vitro reconstitution of microsporidian iron-sulfur cluster biosynthesis
- PMID: 28051091
- PMCID: PMC5216125
- DOI: 10.1038/ncomms13932
Evolutionary conservation and in vitro reconstitution of microsporidian iron-sulfur cluster biosynthesis
Abstract
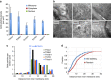
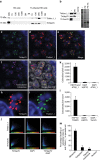
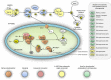
Microsporidians are obligate intracellular parasites that have minimized their genome content and sub-cellular structures by reductive evolution. Here, we demonstrate that cristae-deficient mitochondria (mitosomes) of Trachipleistophora hominis are the functional site of iron-sulfur cluster (ISC) assembly, which we suggest is the essential task of these organelles. Cell fractionation, fluorescence imaging and immunoelectron microscopy demonstrate that mitosomes contain a complete pathway for [2Fe-2S] cluster biosynthesis that we biochemically reconstituted using purified mitosomal ISC proteins. The T. hominis cytosolic iron-sulfur protein assembly (CIA) pathway includes the essential Cfd1-Nbp35 scaffold complex that assembles a [4Fe-4S] cluster as shown by spectroscopic methods in vitro. Phylogenetic analyses reveal that the ISC and CIA pathways are predominantly bacterial, but their cytosolic and nuclear target Fe/S proteins are mainly archaeal. This mixed evolutionary history of Fe/S-related proteins and pathways, and their strong conservation among highly reduced parasites, provides compelling evidence for the ancient chimeric ancestry of eukaryotes.
Figures



References
-
- Vavra J. & Lukes J. Microsporidia and ‘the art of living together'. Adv. Parasitol. 82, 253–319 (2013). - PubMed
-
- Williams B. A., Hirt R. P., Lucocq J. M. & Embley T. M. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 418, 865–869 (2002). - PubMed
-
- Katinka M. D. et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414, 450–453 (2001). - PubMed
-
- Embley T. M. & Martin W. Eukaryotic evolution, changes and challenges. Nature 440, 623–630 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

