Novel Therapeutics Identification for Fibrosis in Renal Allograft Using Integrative Informatics Approach
- PMID: 28051114
- PMCID: PMC5209709
- DOI: 10.1038/srep39487
Novel Therapeutics Identification for Fibrosis in Renal Allograft Using Integrative Informatics Approach
Abstract
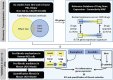
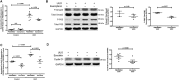
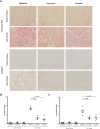
Chronic allograft damage, defined by interstitial fibrosis and tubular atrophy (IF/TA), is a leading cause of allograft failure. Few effective therapeutic options are available to prevent the progression of IF/TA. We applied a meta-analysis approach on IF/TA molecular datasets in Gene Expression Omnibus to identify a robust 85-gene signature, which was used for computational drug repurposing analysis. Among the top ranked compounds predicted to be therapeutic for IF/TA were azathioprine, a drug to prevent acute rejection in renal transplantation, and kaempferol and esculetin, two drugs not previously described to have efficacy for IF/TA. We experimentally validated the anti-fibrosis effects of kaempferol and esculetin using renal tubular cells in vitro and in vivo in a mouse Unilateral Ureteric Obstruction (UUO) model. Kaempferol significantly attenuated TGF-β1-mediated profibrotic pathways in vitro and in vivo, while esculetin significantly inhibited Wnt/β-catenin pathway in vitro and in vivo. Histology confirmed significantly abrogated fibrosis by kaempferol and esculetin in vivo. We developed an integrative computational framework to identify kaempferol and esculetin as putatively novel therapies for IF/TA and provided experimental evidence for their therapeutic activities in vitro and in vivo using preclinical models. The findings suggest that both drugs might serve as therapeutic options for IF/TA.
Figures



References
-
- Meier-Kriesche H. U., Schold J. D. & Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 4, 1289–1295, doi: 10.1111/j.1600-6143.2004.00515.x (2004). - DOI - PubMed
-
- Hariharan S., Alexander J. W., Schroeder T. J. & First M. R. Impact of first acute rejection episode and severity of rejection on cadaveric renal allograft survival. Clinical transplantation 10, 538–541 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

