The larvicide pyriproxyfen blamed during the Zika virus outbreak does not cause microcephaly in zebrafish embryos
- PMID: 28051181
- PMCID: PMC5209666
- DOI: 10.1038/srep40067
The larvicide pyriproxyfen blamed during the Zika virus outbreak does not cause microcephaly in zebrafish embryos
Abstract
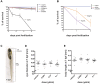
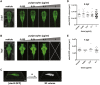
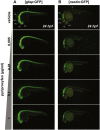
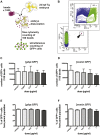
Although the zika virus (ZIKV) has now been strongly correlated with emerging cases of microcephaly in the Americas, suspicions have been raised regarding the use of pyriproxyfen, a larvicide that prevents mosquito development, in drinking water. The effects of this compound on neurodevelopment have not yet been addressed specifically in vertebrates. As a result, we aimed at addressing the effects, if any, of pyriproxyfen on neurodevelopment in the zebrafish embryo as a vertebrate model. Using zebrafish transgenic lines expressing GFP in different cell populations (elavl3 in newborn neurons, gfap and nestin in neural stem cells), we focused on the analysis of whole embryonic brain volume after confocal 3D-reconstruction and the quantification of purified neural stem cells during early neurodevelopment by FACS-cell sorting from whole in vivo embryos. Interestingly, though lethal at very high doses, pyriproxyfen did not cause brain malformation nor any significant changes in the number of observed stem cells in the developing central nervous system. Our data indicate that pyriproxyfen does not affect central nervous system development in zebrafish, suggesting that this larvicide on its own, may not be correlated with the increase in microcephaly cases reported recently.
Figures

References
-
- Corona-Rivera J. R. et al. Report and review of the fetal brain disruption sequence. Eur J Pediatr 160, 664–667 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

