PREX1 integrates G protein-coupled receptor and phosphoinositide 3-kinase signaling to promote glioblastoma invasion
- PMID: 28051998
- PMCID: PMC5352422
- DOI: 10.18632/oncotarget.14348
PREX1 integrates G protein-coupled receptor and phosphoinositide 3-kinase signaling to promote glioblastoma invasion
Abstract
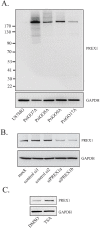
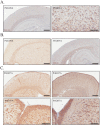
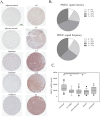
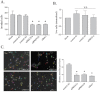
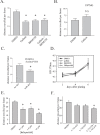
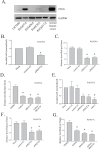
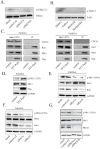
A defining feature of the brain cancer glioblastoma is its highly invasive nature. When glioblastoma cells are isolated from patients using serum free conditions, they accurately recapitulate this invasive behaviour in animal models. The Rac subclass of Rho GTPases has been shown to promote invasive behaviour in glioblastoma cells isolated in this manner. However the guanine nucleotide exchange factors responsible for activating Rac in this context have not been characterized previously. PREX1 is a Rac guanine nucleotide exchange factor that is synergistically activated by binding of G protein αγ subunits and the phosphoinositide 3-kinase pathway second messenger phosphatidylinositol 3,4,5 trisphosphate. This makes it of particular interest in glioblastoma, as the phosphoinositide 3-kinase pathway is aberrantly activated by mutation in almost all cases. We show that PREX1 is expressed in glioblastoma cells isolated under serum-free conditions and in patient biopsies. PREX1 promotes the motility and invasion of glioblastoma cells, promoting Rac-mediated activation of p21-associated kinases and atypical PKC, which have established roles in cell motility. Glioblastoma cell motility was inhibited by either inhibition of phosphoinositide 3-kinase or inhibition of G protein βγ subunits. Motility was also inhibited by the generic dopamine receptor inhibitor haloperidol or a combination of the selective dopamine receptor D2 and D4 inhibitors L-741,626 and L-745,870. This establishes a role for dopamine receptor signaling via G protein βγ subunits in glioblastoma invasion and shows that phosphoinositide 3-kinase mutations in glioblastoma require a context of basal G protein-coupled receptor activity in order to promote this invasion.
Keywords: Abbreviations: GEF; guanine nucleotide exchange factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004;64:8271–8275. - PubMed
-
- Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, Symons M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–7829. - PubMed
-
- Kwiatkowska A, Symons M. Signaling determinants of glioma cell invasion. Adv Exp Med Biol. 2013;986:121–141. - PubMed
-
- Ruiz-Ontanon P, Orgaz JL, Aldaz B, Elosegui-Artola A, Martino J, Berciano MT, Montero JA, Grande L, Nogueira L, Diaz-Moralli S, Esparis-Ogando A, Vazquez-Barquero A, Lafarga M, et al. Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cells. 2013;31:1075–1085. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

