Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages
- PMID: 28052009
- PMCID: PMC5352428
- DOI: 10.18632/oncotarget.14374
Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages
Abstract
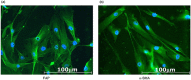
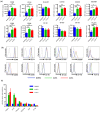
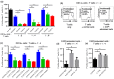
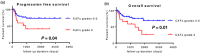
Stromal cells in the tumor microenvironment (TME) closely interact with tumor cells and affect tumor cell behavior in diverse manners. We herein investigated the mechanisms by which cancer-associated fibroblasts (CAFs) affect the functional polarization of tumor-associated macrophages (TAMs) in oral squamous cell carcinoma (OSCC) in vitro and in human cancer samples. The expression of CD68, CD14, CD163, CD200R, CD206, HLA-G, CD80, and CD86 was higher in CD14-positive cells co-cultured with the culture supernatants of CAFs established from OSCC specimens (CAF-educated cells) than in control cells. The gene expression level of ARG1, IL10, and TGFB1 was increased in CAF-educated cells. CAF-educated cells suppressed T cell proliferation more strongly than control cells, and the neutralization of TGF-β IL-10, or arginase I significantly restored T cell proliferation. We then investigated the relationship between the infiltration of CAFs and TAMs using tissue samples obtained from patients with OSCC. The infiltration of CAFs was associated with the numbers of CD68-positive and CD163-positive macrophages. It also correlated with lymphatic invasion, vascular invasion, lymph node involvement, and the TNM stage. The infiltration of CAFs was identified as an independent prognostic factor in OSCC. Our results indicate that CAFs play important roles in shaping the tumor immunosuppressive microenvironment in OSCC by inducing the protumoral phenotype of TAMs. Therapeutic strategies to reverse CAF-mediated immunosuppression need to be considered.
Keywords: cancer-associated fibroblast (CAF); immunomodulation; immunosuppression; tumor microenvironment (TME); tumor-associated macrophage (TAM).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Diminished CD68+ Cancer-Associated Fibroblast Subset Induces Regulatory T-Cell (Treg) Infiltration and Predicts Poor Prognosis of Oral Squamous Cell Carcinoma Patients.Am J Pathol. 2020 Apr;190(4):886-899. doi: 10.1016/j.ajpath.2019.12.007. Epub 2020 Feb 5. Am J Pathol. 2020. PMID: 32035062
-
Signal regulatory protein α associated with the progression of oral leukoplakia and oral squamous cell carcinoma regulates phenotype switch of macrophages.Oncotarget. 2016 Dec 6;7(49):81305-81321. doi: 10.18632/oncotarget.12874. Oncotarget. 2016. PMID: 27793032 Free PMC article.
-
Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs.Sci Rep. 2019 Feb 28;9(1):3172. doi: 10.1038/s41598-019-39553-z. Sci Rep. 2019. PMID: 30816272 Free PMC article.
-
Role of tumour-associated macrophages in oral squamous cells carcinoma progression: an update on current knowledge.Diagn Pathol. 2017 Apr 5;12(1):32. doi: 10.1186/s13000-017-0623-6. Diagn Pathol. 2017. PMID: 28381274 Free PMC article. Review.
-
Tumor microenvironment in oral squamous cell carcinoma.Front Immunol. 2024 Dec 18;15:1485174. doi: 10.3389/fimmu.2024.1485174. eCollection 2024. Front Immunol. 2024. PMID: 39744628 Free PMC article. Review.
Cited by
-
Arginase-1 in Plasma-Derived Exosomes as Marker of Metastasis in Patients with Head and Neck Squamous Cell Carcinoma.Cancers (Basel). 2023 Nov 16;15(22):5449. doi: 10.3390/cancers15225449. Cancers (Basel). 2023. PMID: 38001706 Free PMC article.
-
The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment.Front Immunol. 2019 Aug 2;10:1835. doi: 10.3389/fimmu.2019.01835. eCollection 2019. Front Immunol. 2019. PMID: 31428105 Free PMC article. Review.
-
In search of definitions: Cancer-associated fibroblasts and their markers.Int J Cancer. 2020 Feb 15;146(4):895-905. doi: 10.1002/ijc.32193. Epub 2019 Feb 28. Int J Cancer. 2020. PMID: 30734283 Free PMC article. Review.
-
EVA1C Is a Potential Prognostic Biomarker and Correlated With Immune Infiltration Levels in WHO Grade II/III Glioma.Front Immunol. 2021 Jun 29;12:683572. doi: 10.3389/fimmu.2021.683572. eCollection 2021. Front Immunol. 2021. PMID: 34267752 Free PMC article.
-
Forces exerted and transduced by cancer-associated fibroblasts during cancer progression.Biol Cell. 2023 Aug;115(8):e2200104. doi: 10.1111/boc.202200104. Epub 2023 Jun 12. Biol Cell. 2023. PMID: 37224184 Free PMC article. Review.
References
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
-
- Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669–682. - PubMed
-
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

