A screen for Fli-1 transcriptional modulators identifies PKC agonists that induce erythroid to megakaryocytic differentiation and suppress leukemogenesis
- PMID: 28052010
- PMCID: PMC5369997
- DOI: 10.18632/oncotarget.14377
A screen for Fli-1 transcriptional modulators identifies PKC agonists that induce erythroid to megakaryocytic differentiation and suppress leukemogenesis
Abstract
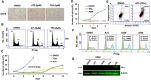
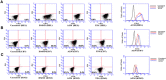
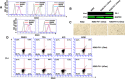
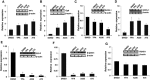
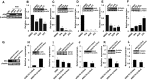
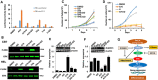
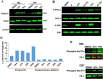
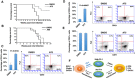
The ETS-related transcription factor Fli-1 affects many developmental programs including erythroid and megakaryocytic differentiation, and is frequently de-regulated in cancer. Fli-1 was initially isolated following retrovirus insertional mutagenesis screens for leukemic initiator genes, and accordingly, inhibition of this transcription factor can suppress leukemia through induction of erythroid differentiation. To search for modulators of Fli-1, we hereby performed repurposing drug screens with compounds isolated from Chinese medicinal plants. We identified agents that can transcriptionally activate or inhibit a Fli-1 reporter. Remarkably, agents that increased Fli-1 transcriptional activity conferred a strong anti-cancer activity upon Fli-1-expressing leukemic cells in culture. As opposed to drugs that suppress Fli1 activity and lead to erythroid differentiation, growth suppression by these new Fli-1 transactivating compounds involved erythroid to megakaryocytic conversion (EMC). The identified compounds are structurally related to diterpene family of small molecules, which are known agonists of protein kinase C (PKC). In accordance, these PKC agonists (PKCAs) induced PKC phosphorylation leading to activation of the mitogen-activated protein kinase (MAPK) pathway, increased cell attachment and EMC, whereas pharmacological inhibition of PKC or MAPK diminished the effect of our PKCAs. Moreover, in a mouse model of leukemia initiated by Fli-1 activation, the PKCA compounds exhibited strong anti-cancer activity, which was accompanied by increased presence of CD41/CD61 positive megakaryocytic cells in leukemic spleens. Thus, PKC agonists offer a novel approach to combat Fli-1-induced leukemia, and possibly other cancers,by inducing EMC in part through over-activation of the PKC-MAPK-Fli-1 pathway.
Keywords: Fli-1; PKC; drug screens; erythroid and megakaryocytic differentiation; leukemia therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Fli-1 overexpression in erythroleukemic cells promotes erythroid de-differentiation while Spi-1/PU.1 exerts the opposite effect.Int J Oncol. 2017 Aug;51(2):456-466. doi: 10.3892/ijo.2017.4027. Epub 2017 Jun 2. Int J Oncol. 2017. PMID: 28586009 Free PMC article.
-
Selective ERK1/2 agonists isolated from Melia azedarach with potent anti-leukemic activity.BMC Cancer. 2019 Aug 2;19(1):764. doi: 10.1186/s12885-019-5914-8. BMC Cancer. 2019. PMID: 31375085 Free PMC article.
-
FLI-1 is a suppressor of erythroid differentiation in human hematopoietic cells.Leukemia. 2000 Mar;14(3):439-45. doi: 10.1038/sj.leu.2401689. Leukemia. 2000. PMID: 10720139
-
Current insights into the role of Fli-1 in hematopoiesis and malignant transformation.Cell Mol Life Sci. 2022 Feb 28;79(3):163. doi: 10.1007/s00018-022-04160-1. Cell Mol Life Sci. 2022. PMID: 35412146 Free PMC article. Review.
-
Anthracyclines as tumor cell differentiating agents: effects on the regulation of erythroid gene expression.Leuk Lymphoma. 1997 Aug;26(5-6):575-87. doi: 10.3109/10428199709050893. Leuk Lymphoma. 1997. PMID: 9389364 Review.
Cited by
-
FLI1 Induces Megakaryopoiesis Gene Expression Through WAS/WIP-Dependent and Independent Mechanisms; Implications for Wiskott-Aldrich Syndrome.Front Immunol. 2021 Feb 26;12:607836. doi: 10.3389/fimmu.2021.607836. eCollection 2021. Front Immunol. 2021. PMID: 33717090 Free PMC article.
-
FLI1 promotes protein translation via the transcriptional regulation of MKNK1 expression.Int J Oncol. 2020 Feb;56(2):430-438. doi: 10.3892/ijo.2019.4943. Epub 2019 Dec 16. Int J Oncol. 2020. PMID: 31894299 Free PMC article.
-
A novel 3',5'-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCδ/JNK signal in prostate cancer.Aging (Albany NY). 2020 May 19;12(10):9103-9124. doi: 10.18632/aging.103178. Epub 2020 May 19. Aging (Albany NY). 2020. PMID: 32427575 Free PMC article.
-
Vitamin D3 and its active form calcitriol suppress erythroleukemia through upregulation of CHAC1 and downregulation of NOTCH1.Med Oncol. 2025 Mar 27;42(5):138. doi: 10.1007/s12032-025-02695-4. Med Oncol. 2025. PMID: 40146328
-
Identification of diterpenoid compounds that interfere with Fli-1 DNA binding to suppress leukemogenesis.Cell Death Dis. 2019 Feb 11;10(2):117. doi: 10.1038/s41419-019-1363-1. Cell Death Dis. 2019. PMID: 30741932 Free PMC article.
References
-
- Johnston SJ, Carroll JS. Transcription factors and chromatin proteins as therapeutic targets in cancer. Biochim Biophys Acta. 2015;1855:183–92. - PubMed
-
- Ben-David Y, Giddens EB, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–18. - PubMed
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, Et A. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

