Genome-wide in vivo screen identifies novel host regulators of metastatic colonization
- PMID: 28052056
- PMCID: PMC5603286
- DOI: 10.1038/nature20792
Genome-wide in vivo screen identifies novel host regulators of metastatic colonization
Abstract
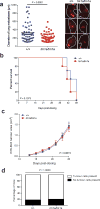
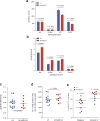
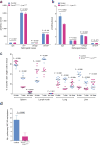
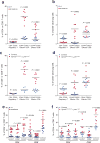
Metastasis is the leading cause of death for cancer patients. This multi-stage process requires tumour cells to survive in the circulation, extravasate at distant sites, then proliferate; it involves contributions from both the tumour cell and tumour microenvironment ('host', which includes stromal cells and the immune system). Studies suggest the early steps of the metastatic process are relatively efficient, with the post-extravasation regulation of tumour growth ('colonization') being critical in determining metastatic outcome. Here we show the results of screening 810 mutant mouse lines using an in vivo assay to identify microenvironmental regulators of metastatic colonization. We identify 23 genes that, when disrupted in mouse, modify the ability of tumour cells to establish metastatic foci, with 19 of these genes not previously demonstrated to play a role in host control of metastasis. The largest reduction in pulmonary metastasis was observed in sphingosine-1-phosphate (S1P) transporter spinster homologue 2 (Spns2)-deficient mice. We demonstrate a novel outcome of S1P-mediated regulation of lymphocyte trafficking, whereby deletion of Spns2, either globally or in a lymphatic endothelial-specific manner, creates a circulating lymphopenia and a higher percentage of effector T cells and natural killer (NK) cells present in the lung. This allows for potent tumour cell killing, and an overall decreased metastatic burden.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



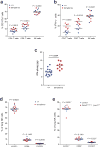




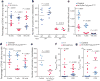
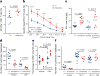
Comment in
-
Mouse genomic screen reveals novel host regulator of metastasis.Genome Biol. 2017 Feb 16;18(1):31. doi: 10.1186/s13059-017-1170-x. Genome Biol. 2017. PMID: 28209174 Free PMC article.
-
When metastasis 'Spns' out of control: Coverage of 'Genome-wide in vivo screen identifies novel host regulators of metastatic colonization'.Pigment Cell Melanoma Res. 2017 Jul;30(4):384-385. doi: 10.1111/pcmr.12588. Epub 2017 Jun 13. Pigment Cell Melanoma Res. 2017. PMID: 28299902 No abstract available.
References
-
- Chambers AF, et al. Critical steps in hematogenous metastasis: an overview. Surg. Oncol. Clin. N. Am. 2001;10:243–255. - PubMed
-
- Blank C, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. - PubMed
-
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

