Defining Surrogate Endpoints for Clinical Trials in Severe Falciparum Malaria
- PMID: 28052109
- PMCID: PMC5215574
- DOI: 10.1371/journal.pone.0169307
Defining Surrogate Endpoints for Clinical Trials in Severe Falciparum Malaria
Abstract
Background: Clinical trials in severe falciparum malaria require a large sample size to detect clinically meaningful differences in mortality. This means few interventions can be evaluated at any time. Using a validated surrogate endpoint for mortality would provide a useful alternative allowing a smaller sample size. Here we evaluate changes in coma score and plasma lactate as surrogate endpoints for mortality in severe falciparum malaria.
Methods: Three datasets of clinical studies in severe malaria were re-evaluated: studies from Chittagong, Bangladesh (adults), the African 'AQUAMAT' trial comparing artesunate and quinine (children), and the Vietnamese 'AQ' study (adults) comparing artemether with quinine. The absolute change, relative change, slope of the normalization over time, and time to normalization were derived from sequential measurements of plasma lactate and coma score, and validated for their use as surrogate endpoint, including the proportion of treatment effect on mortality explained (PTE) by these surrogate measures.
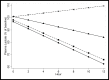
Results: Improvements in lactate concentration or coma scores over the first 24 hours of admission, were strongly prognostic for survival in all datasets. In hyperlactataemic patients in the AQ study (n = 173), lower mortality with artemether compared to quinine closely correlated with faster reduction in plasma lactate concentration, with a high PTE of the relative change in plasma lactate at 8 and 12 hours of 0.81 and 0.75, respectively. In paediatric patients enrolled in the 'AQUAMAT' study with cerebral malaria (n = 785), mortality was lower with artesunate compared to quinine, but this was not associated with faster coma recovery.
Conclusions: The relative changes in plasma lactate concentration assessed at 8 or 12 hours after admission are valid surrogate endpoints for severe malaria studies on antimalarial drugs or adjuvant treatments aiming at improving the microcirculation. Measures of coma recovery are not valid surrogate endpoints for mortality.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Atta H, Reeder J. World Malaria Day 2014: invest in the future. Defeat malaria. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2014;20(4):219–20. - PubMed
-
- Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;47(2):151–7. - PubMed
-
- von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, et al. Predicting the clinical outcome of severe falciparum malaria in african children: findings from a large randomized trial. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(8):1080–90. - PMC - PubMed
-
- Severe malaria. Tropical medicine & international health: TM & IH. 2014;19 Suppl 1:7–131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

