Axillary treatment for operable primary breast cancer
- PMID: 28052186
- PMCID: PMC6464919
- DOI: 10.1002/14651858.CD004561.pub3
Axillary treatment for operable primary breast cancer
Abstract
Background: Axillary surgery is an established part of the management of primary breast cancer. It provides staging information to guide adjuvant therapy and potentially local control of axillary disease. Several alternative approaches to axillary surgery are available, most of which aim to spare a proportion of women the morbidity of complete axillary dissection.
Objectives: To assess the benefits and harms of alternative approaches to axillary surgery (including omitting such surgery altogether) in terms of overall survival; local, regional and distant recurrences; and adverse events.
Search methods: We searched the Cochrane Breast Cancer Group Specialised Register, MEDLINE, Pre-MEDLINE, Embase, CENTRAL, the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov on 12 March 2015 without language restrictions. We also contacted study authors and checked reference lists.
Selection criteria: Randomised controlled trials (RCTs) including women with clinically defined operable primary breast cancer conducted to compare axillary lymph node dissection (ALND) with no axillary surgery, axillary sampling or sentinel lymph node biopsy (SLNB); RCTs comparing axillary sampling with SLNB or no axillary surgery; RCTs comparing SLNB with no axillary surgery; and RCTs comparing ALND with or without radiotherapy (RT) versus RT alone.
Data collection and analysis: Two review authors independently assessed each potentially relevant trial for inclusion. We independently extracted outcome data, risk of bias information and study characteristics from all included trials. We pooled data according to trial interventions, and we used hazard ratios (HRs) for time-to-event outcomes and odds ratios (OR) for binary outcomes.
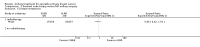
Main results: We included 26 RCTs in this review. Studies were at low or unclear risk of selection bias. Blinding was not done, but this was only considered a source of bias for outcomes with potential for subjectivity in measurements. We found no RCTs of axillary sampling versus SLNB, axillary sampling versus no axillary surgery or SLNB versus no axillary surgery. No axillary surgery versus ALND Ten trials involving 3849 participants compared no axillary surgery versus ALND. Moderate quality evidence showed no important differences between overall survival of women in the two groups (HR 1.06, 95% confidence interval (CI) 0.96 to 1.17; 3849 participants; 10 studies) although no axillary surgery increased the risk of locoregional recurrence (HR ranging from 1.10 to 3.06; 20,863 person-years of follow-up; four studies). It was uncertain whether no surgery increased the risk of distant metastasis compared with ALND (HR 1.06, 95% CI 0.87 to 1.30; 946 participants; two studies). Low-quality evidence indicated no axillary surgery decreased the risk of lymphoedema compared with ALND (OR 0.31, 95% CI 0.23 to 0.43; 1714 participants; four studies). Axillary sampling versus ALND Six trials involving 1559 participants compared axillary sampling versus ALND. Low-quality evidence indicated similar effectiveness of axillary sampling compared with ALND in terms of overall survival (HR 0.94, 95% CI 0.73 to 1.21; 967 participants; three studies) but it was unclear whether axillary sampling led to increased risk of local recurrence compared with ALND (HR 1.41, 95% CI 0.94 to 2.12; 1404 participants; three studies). The relative effectiveness of axillary sampling and ALND for locoregional recurrence (HR 0.74, 95% CI 0.46 to 1.20; 406 participants; one study) and distant metastasis was uncertain (HR 1.05, 95% CI 0.74 to 1.49; 406 participants; one study). Lymphoedema was less likely after axillary sampling than after ALND (OR 0.32, 95% CI 0.13 to 0.81; 80 participants; one study). SLNB versus ALND Seven trials involving 9426 participants compared SLNB with ALND. Moderate-quality evidence showed similar overall survival following SLNB compared with ALND (HR 1.05, 95% CI 0.89 to 1.25; 6352 participants; three studies; moderate-quality evidence). Differences in local recurrence (HR 0.94, 95% CI 0.24 to 3.77; 516 participants; one study), locoregional recurrence (HR 0.96, 95% CI 0.74 to 1.24; 5611 participants; one study) and distant metastasis (HR 0.80, 95% CI 0.42 to 1.53; 516 participants; one study) were uncertain. However, studies showed little absolute difference in the aforementioned outcomes. Lymphoedema was less likely after SLNB than ALND (OR ranged from 0.04 to 0.60; three studies; 1965 participants; low-quality evidence). Three studies including 1755 participants reported quality of life: Investigators in two studies found quality of life better after SLNB than ALND, and in the other study observed no difference. RT versus ALND Four trials involving 2585 participants compared RT alone with ALND (with or without RT). High-quality evidence indicated that overall survival was reduced among women treated with radiotherapy alone compared with those treated with ALND (HR 1.10, 95% CI 1.00 to 1.21; 2469 participants; four studies), and local recurrence was less likely in women treated with radiotherapy than in those treated with ALND (HR 0.80, 95% CI 0.64 to 0.99; 22,256 person-years of follow-up; four studies). Risk of distant metastasis was similar for radiotherapy alone as for ALND (HR 1.07, 95% CI 0.93 to 1.25; 1313 participants; one study), and whether lymphoedema was less likely after RT alone than ALND remained uncertain (OR 0.47, 95% CI 0.16 to 1.44; 200 participants; one study). Less surgery versus ALND When combining results from all trials, treatment involving less surgery was associated with reduced overall survival compared with ALND (HR 1.08, 95% CI 1.01 to 1.17; 6478 participants; 18 studies). Whether local recurrence was reduced with less axillary surgery when compared with ALND was uncertain (HR 0.90, 95% CI 0.75 to 1.09; 24,176 participant-years of follow up; eight studies). Locoregional recurrence was more likely with less surgery than with ALND (HR 1.53, 95% CI 1.31 to 1.78; 26,880 participant-years of follow-up; seven studies). Whether risk of distant metastasis was increased after less axillary surgery compared with ALND was uncertain (HR 1.07, 95% CI 0.95 to 1.20; 2665 participants; five studies). Lymphoedema was less likely after less axillary surgery than with ALND (OR 0.37, 95% CI 0.29 to 0.46; 3964 participants; nine studies).No studies reported on disease control in the axilla.
Authors' conclusions: This review confirms the benefit of SLNB and axillary sampling as alternatives to ALND for axillary staging, supporting the view that ALND of the clinically and radiologically uninvolved axilla is no longer acceptable practice in people with breast cancer.
Conflict of interest statement
None known.
Figures

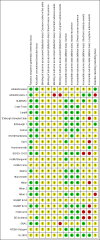



















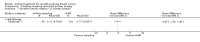








































Update of
References
References to studies included in this review
Addenbrookes {published data only}
Addenbrookes 2 {published data only}
-
- Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. Journal of Clinical Oncology 2005;23(19):4312‐21. - PubMed
ALMANAC {published data only}
-
- Clarke D, Mansel RE. The learning curve in sentinel node biopsy (SNB) in breast cancer: results from the ALMANAC trial. Breast Cancer Research and Treatment. 69 2001; Vol. 69, issue 3:212.
-
- Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post‐operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Research and Treatment 2006;95:279‐93. - PubMed
-
- Mansel RE. The ALMANAC trial: initial experience. Breast Cancer Research and Treatment. 64 2000; Vol. 64, issue 1:36.
-
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. Journal of the National Cancer Institute 2006;98(9):599‐609. - PubMed
-
- Mansel RE, Goyal A, Fallowfield L, Newcombe RG. Sentinel node biopsy in breast cancer: the first results of the randomized multicenter ALMANAC trial. Journal of Clinical Oncology 2004;22(14):4S.
Cape Town {published data only}
-
- Dent DM, Gudgeon CA, Murray EM. Mastectomy with axillary clearance versus mastectomy without it. Late results of a trial in which patients had no adjuvant chemo‐, radio‐ or endocrine therapy. South African Medical Journal 1996;86(6):670‐1. - PubMed
-
- Helman P, Bennett MB, Louw JH, Wilkie W, Madden P, Silber W, et al. Interim report on trial of treatment for operable breast cancer. South African Medical Journal 1972;46(38):1374‐5. - PubMed
Cardiff {published data only}
-
- Forrest AP, Roberts MM, Cant EL, Henk M, Hughes LE, Hulbert M, et al. Simple mastectomy and pectoral node biopsy: the Cardiff St. Mary's trial. World Journal of Surgery 1977;1(3):320‐3. - PubMed
-
- Forrest AP, Roberts MM, Preece P, Henk JM, Campbell H, Hughes LE, et al. The Cardiff‐St Mary's trial. British Journal of Surgery 1974;61(10):766‐9. - PubMed
-
- Roberts MM, Blumgart LH, Davies M, Henk JM, Forrest AP, Campbell H, et al. Simple versus radical mastectomy. Preliminary report of the Cardiff breast trial. Lancet 1973;1(812):1073‐6. - PubMed
-
- Roberts MM, Davies M, Henk M, Forrest AP, Blumgart LH, Gleave EN, et al. A clinical trial of treatment in early breast cancer. British Journal of Surgery 1973;60(4):305‐6. - PubMed
E'dburgh Sample/Clear {published data only}
-
- Aitken RJ, Gaze MN, Rodger A, Chetty U, Forrest AP. Arm morbidity within a trial of mastectomy and either nodal sample with selective radiotherapy or axillary clearance. British Journal of Surgery 1989;76(6):568‐71. - PubMed
-
- Forrest AP, Everington D, McDonald CC, Steele RJ, Chetty U, Stewart HJ. The Edinburgh randomized trial of axillary sampling or clearance after mastectomy. British Journal of Surgery 1995;82(11):1504‐8. [MEDLINE: ] - PubMed
-
- Lambah PA, Dixon JM, Prescott RJ, Forrest JW. Long term results of randomized studies of axillary clearance vs non‐targeted axillary sampling. Breast Cancer Research & Treatment. 64 2000; Vol. 64, issue 1:38. [MEDLINE: ]
-
- Steele R, Forrest A, Gibson T, Stewart H, Chetty U. The efficacy of lower axillary sampling in obtaining lymph node status in breast cancer: a controlled randomized trial. British Journal of Surgery 1985;72:368‐9. [MEDLINE: ] - PubMed
Edinburgh 1 {published data only}
-
- Chetty U, Jack W, Prescott RJ, Tyler C, Rodger A. Management of the axilla in operable breast cancer treated by breast conservation: a randomized clinical trial. Edinburgh Breast Unit. British Journal of Surgery 2000;87(2):163‐9. - PubMed
Genoa {published data only}
-
- Canavese G, Catturich A, Vecchio C, Tomei D, Gipponi M, Villa G, et al. Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: results of randomized trial. Annals of Oncology 2009;20(6):1001‐7. - PubMed
GIVOM Sentinella {published data only}
-
- Bianco P, Zavagno G, Burelli P, Scalco G, Barutta L, Carraro P, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella‐GIVOM Italian randomised clinical trial. European Journal of Surgical Oncology 2008;34(5):508‐13. - PubMed
-
- Zavagno G. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the sentinella/GIVOM trial. Annals of Surgery 2008;247(2):207‐13. - PubMed
-
- Zavagno G. Clinical impact of false‐negative sentinel lymph nodes in breast cancer. European Journal of Surgical Oncology 2008;34(6):620‐5. - PubMed
Guy's {published data only}
-
- Fentiman IS. Long‐term follow‐up of the first breast conservation trial: Guy's wide excision study. Breast 2000;9:1‐8. [MEDLINE: ] - PubMed
-
- Hayward J. Conservative surgery in the treatment of early breast cancer. British Journal of Surgery 1974;61(10):770‐1. - PubMed
-
- Hayward JL. The Guy's trial of treatments of 'early' breast cancer. World Journal of Surgery 1977;1:314‐6. - PubMed
Hammersmith {published data only}
-
- Burn JI. 'Early' breast cancer: the Hammersmith trial. An interim report. British Journal of Surgery 1974;61(10):762‐5. - PubMed
-
- Burn JI, Davey J, Sellwood RA, Deeley TJ, Azzopardi J, Li J. A prospective comparative clinical trial in early carcinoma of the female breast. British Journal of Surgery 1968;55(11):869. - PubMed
IBCSG‐10‐93 {published data only}
-
- Goldhirsch A, Gelber RD, Castiglione M, Price KN, Rudenstam CM, Lindtner J, et al. The best available adjuvant treatments are within the framework of clinical trials. The International Breast Cancer Study Group. Israel Journal of Medical Sciences 1995;31(2‐3):144‐54. - PubMed
-
- International Breast Cancer Study Group, Rudenstam CM, Zahrieh D, Forbes JF, Crivellari D, Holmberg SB, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10‐93 [see comment]. Journal of Clinical Oncology 2006;24(3):337‐44. - PubMed
Institut Bergonie {published data only}
-
- Avril A, Bouedec G, Lorimier G, Classe JM, Tunon‐de‐Lara C, Giard S, et al. Phase III randomized equivalence trial of early breast cancer treatments with or without axillary clearance in post‐menopausal patients: results after 5 years of follow‐up. European Journal of Surgical Oncology 2011;37:563‐70. - PubMed
Institut Curie {published data only}
-
- Cabanes PA, Salmon RJ, Vilcoq JR, Durand JC, Fourquet A, Gautier C, et al. Value of axillary dissection in addition to lumpectomy and radiotherapy in early breast cancer. The Breast Carcinoma Collaborative Group of the Institut Curie [see comments]. Lancet 1992;339(8804):1245‐8. - PubMed
-
- Louis‐Sylvestre C, Clough K, Asselain B, Vilcoq JR, Salmon RM, Campana F, et al. Axillary treatment in conservative management operable breast cancer: dissection or radiotherapy? Results of a randomised study with 15 years of follow‐up. Journal of Clinical Oncology 2004;22(1):97‐101. - PubMed
-
- Louis‐Sylvestre C, Clough KB, Falcou M‐C, Salmon RJ, Fourquet A, Vilcoq JR. A randomized trial comparing axillary dissection and axillary radiotherapy for early breast cancer: 15 year results. Breast Cancer Research and Treatment. 69 2001; Vol. 69, issue 3:212.
-
- Salmon RJ, Vilcoq JR, Durand JC, Cabanes PA, Asselain B. Lumpectomy with or without axillary dissection for early breast cancer: results of a prospective randomised trial. Breast Cancer Research and Treatment. 19 1991; Vol. 19, issue 2:212.
Malmo {published data only}
-
- Borgstrom S, Linell F, Tennvall‐Nittby L, Ranstam J, Tennvall Nittby L. Mastectomy only versus radical mastectomy and postoperative radiotherapy in node negative, resectable breast cancer. A randomized trial. Acta Oncologica 1994;33(5):557‐60. - PubMed
Manchester {published data only}
Milan {published data only}
-
- Veronesi U. Sentinel lymph node biopsy in breast cancer: ten‐year results of a randomized controlled study. Annals of Surgery 2010;251(4):595‐600. - PubMed
-
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel‐node biopsy with routine axillary dissection in breast cancer [comment]. New England Journal of Medicine 2003;349(6):546‐53. [MEDLINE: ] - PubMed
Milan 2 {published data only}
-
- Martelli G, Boracchi P, Ardoino I, Lozza L, Bohm S, Vetrella G, et al. Axillary dissection versus no axillary dissection in older patients with T0N0 breast cancer: 15‐year results of a randomized controlled trial. Annals of Surgery 2012;256:920‐4. - PubMed
Milan 3 {published data only}
-
- Agresti R. Axillary dissection vs no axillary surgery in T1N0 breast cancer patients: a randomized clinical trial (INT 09/98). European Journal of Cancer. 2013; Vol. Conference:S457.
-
- Agresti R, Martelli G, Menard S, Ferraris C, Maugen I, Pelliteri C, et al. Conservative surgery with or without axillary clearance in T1N0 breast cancer: ten‐year results of INT 09/98 randomised trial. European Journal of Cancer. 2012; Vol. Conference:S45.
-
- Agresti R, Martelli G, Sandri M, Tagliabue E, Carcangiu ML, Maugeri I, et al. Axillary lymph node dissection versus no dissection in patients with T1N0 breast cancer: a randomized clinical trial (INT09/98). Cancer 2014;120:885‐93. - PubMed
NSABP B‐04 {published data only}
-
- Deutsch M, Land S, Begovic M, Sharif S. The incidence of arm edema in women with breast cancer randomized on the National Surgical Adjuvant Breast and Bowel Project study B‐04 to radical mastectomy versus total mastectomy and radiotherapy versus total mastectomy alone. International Journal of Radiation Oncology, Biology, Physics 2008;70:1020‐4. - PubMed
-
- Fisher B. The operative management of primary breast cancer. International Journal of Radiation Oncology, Biology, Physics 1977;2(9‐10):989‐92. [MEDLINE: ] - PubMed
-
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty‐five‐year follow‐up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation [see comment]. New England Journal of Medicine 2002;347(8):567‐75. [MEDLINE: ] - PubMed
-
- Fisher B, Montague E, Redmond C, Barton B, Borland D, Fisher ER, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer 1977;39:2827‐39. [MEDLINE: ] - PubMed
-
- Fisher B, Montague E, Redmond C, Deutsch M, Brown GR, Zauber A, et al. Findings from NSABP protocol No. B‐04: comparison of radical mastectomy with alternative treatments for primary breast cancer. Cancer 1980;46(1):1‐13. [MEDLINE: ] - PubMed
NSABP B‐32 {published data only}
-
- Julian TB, Anderson SJ, Krag DN, Harlow SP, Costantino JP, Ashikaga T, et al. 10‐yr follow‐up results of NSABP‐32, a randomized phase III clinical trial to compare sentinel node resection (SNR) to conventional axillary dissection (AD) in clinically node‐negative breast cancer patients. Journal of Clinical Oncology 2013;31(15 Suppl):1000.
-
- Julian TB, Anderson SJ, Krag DN, Weaver DL, Costantino JP, Ashikaga T, et al. Abstract S2‐05: 10‐yr follow‐up results of occult detected sentinel node disease: NSABP B‐32, a randomized phase III clinical trial to compare sentinel node resection (SNR) to conventional axillary dissection (AD) in clinically node‐negative breast cancer patients. Cancer Research 2013;73:S2‐05.
-
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel‐lymph‐node resection and conventional axillary‐lymph‐node dissection in patients with clinically node‐negative breast cancer: results from the NSABP B‐32 randomised phase III trial.[see comment]. Lancet Oncology 2007;8(10):881‐8. - PubMed
-
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel‐lymph node resection compared with conventional axillary‐lymph node dissection in clinically node‐negative patients with breast cancer: overall survival findings from the NSABPB‐32 randomised phase 3 trial. Lancet Oncology 2010;11:927‐33. - PMC - PubMed
Ostersund {published data only}
-
- Borup CS, Jansson C. Axillary biopsy compared with dissection in the staging of lymph nodes in operable breast cancer. A randomised trial. European Journal of Surgery 1993;159(4):159‐62. - PubMed
-
- Borup Christensen S, Lundgren E. Sequelae of axillary dissection vs. axillary sampling with or without irradiation for breast cancer. A randomized trial. Acta Chirurgica Scandinavica 1989;155(10):515‐9. - PubMed
SE Scotland {published data only}
-
- Bruce J. Operable cancer of the breast. A controlled clinical trial. Cancer 1971;28:1443‐52. - PubMed
-
- Hamilton T, Fraser J, Bruce J. Carcinoma of the breast ‐ a clinical trial. British Journal of Surgery 1969;56(8):615. - PubMed
-
- Hamilton T, Langlands AO. A clinical trial in the management of operable cancer of the breast. Journal of the Royal College of Surgeons of Edinburgh 1977;22(1):52‐5. - PubMed
-
- Hamilton T, Langlands AO, Prescott RJ. The treatment of operable cancer of the breast: a clinical trial in the South‐East region of Scotland. British Journal of Surgery 1974;61(10):758‐61. - PubMed
-
- Langlands AO, Prescott RJ, Hamilton T. A clinical trial in the management of operable cancer of the breast. British Journal of Surgery 1980;67(3):170‐4. - PubMed
SNAC {published data only}
-
- Gill G, SNAC Trial Group of the Royal Australasian College of Surgeons (RACS) and NHMRC Clinical Trials Centre. Sentinel‐lymph‐node‐based management or routine axillary clearance? One‐year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Annals of Surgical Oncology 2009;16(2):266‐75. - PubMed
-
- Smith MJ, Gill PG, Wetzig N, Sourjina T, Gebski V, Ung O, et al. Comparing patients' and clinicians' assessment of outcomes in a randomised trial of sentinel node biopsy for breast cancer (the RACS SNAC trial). Breast Cancer Research & Treatment 2009;117(1):99‐109. - PubMed
-
- Wetzig N, Gill PG, Zannino D, Stockler MR, Gebski V, Ung O, et al. Sentinel lymph node based management or routine axillary clearance? Three‐year outcomes of the RACS Sentinel Node Biopsy Versus Axillary Clearance (SNAC) 1 Trial. Annals of Surgical Oncology 2014;22:17‐23. - PubMed
-
- Wetzig NR, Gill PG, Ung O, Collins J, Kollias J, Gillett D, et al. Participation in the RACS sentinel node biopsy versus axillary clearance trial. ANZ Journal of Surgery 2005; Vol. 75, issue 3:98‐100. - PubMed
WSSA Glasgow {published data only}
-
- Early Breast Cancer Trialists Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. New England Journal of Medicine 1995;333(292):1444‐55. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). West of Scotland study of treatment of mammary carcinoma. Treatment of Early Breast Cancer 1990;1:117.
Xu 2003 {published data only}
-
- Xu H, Zhang B, Zhang Q. The comparison between mastectomy plus axillary dissection and radical mastectomy in patients with I and II stage breast cancer: follow‐up of a randomized controlled study of 192 cases. Chinese Journal of Practical Surgery 2003;23:614‐6.
References to studies excluded from this review
AATRM‐048‐13‐2000 {published data only}
-
- Sola MS, Alberro JA, Fraile M, Santesteban P, Ramos M, Fabregas R, et al. Complete axillary lymph node dissection versus clinical follow‐up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Annals of Surgical Oncology 2013;20:120‐7. - PubMed
ACOSOG Z0011 {published data only}
-
- Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Annals of Surgery 2010;252:426‐32. - PMC - PubMed
-
- Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. Journal of Clinical Oncology 2007;25(24):3657‐63. - PubMed
-
- Olson JA Jr, McCall LM. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group trials Z0010 and Z0011. Journal of Clinical Oncology 2008;26(21):3530‐5. - PubMed
Buenos Aires {published data only}
-
- Gori J, Castano R, Engel H, Toziano M, Fischer C, Maletti G. Conservative treatment vs. mastectomy without radiotherapy in aged women with breast cancer ‐ a prospective and randomized trial. Zentralblatt fur Gynakologie 2000;122(6):311‐7. - PubMed
Copenhagen {published data only}
-
- Johansen H, Kaae S, Schiodt T. Simple mastectomy with postoperative irradiation versus extended radical mastectomy in breast cancer. A twenty‐five‐year follow‐up of a randomized trial. Acta Oncologica 1990;29(6):709‐15. - PubMed
Edinburgh SES {published data only}
-
- Stewart HJ, Jack WJ, Everington D, Forrest AP, Rodger A, McDonald CC, et al. South‐east Scottish trial of local therapy in node negative breast cancer. The Breast 1994;3(1):31‐9.
IBCSG‐23‐01 {published data only}
-
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. S3‐1. Update of International Breast Cancer Study Group Trial 23‐01 to compare axillary dissection versus no axillary dissection in patients with clinically node negative breast cancer and micrometastases in the sentinel node. Cancer Research 2012;71(24 Suppl):S3‐1.
IPO‐P {published data only}
-
- Fougo JL, Dinis‐Ribeiro M, Araujo C, Dias T, Reis P, Giesteira L, et al. Impact of lymphadenectomy on axillary recurrence and morbidity of the upper limb in breast cancer patients with negative sentinel node. A prospective randomised study. Cirugia Espanola 2011;89(5):307‐16. - PubMed
OTOASOR {published data only}
-
- Savolt A, Musonda P, Matrai Z, Polgar C, Renyi‐Vamos F, Rubovszky G, et al. Optimal treatment of the axilla after positive sentinel lymph node biopsy in early invasive breast cancer. Early results of the OTOASOR trial. Orvosi Hetilap 2013;154:1934‐42. - PubMed
-
- Savolt A, Polgar C, Musonda P, Matrai Z, Renyi‐Vamos F, Toth L, et al. Does the result of completion axillary lymph node dissection influence the recommendation for adjuvant treatment in sentinel lymph node‐positive patients?. Clinical Breast Cancer 2013;13:364‐70. - PubMed
References to studies awaiting assessment
Semiglazov 2003 {published data only}
-
- Semiglazov VF, Chagunava OL. Sparing and organ‐saving operations in breast cancer. Voprosy Onkologii 1990;36:535‐9. - PubMed
-
- Semiglazov VF, Maksimov SI, Semiglazov VV, Kosnikov AG. The modern organ‐ and function‐sparing surgical treatment in oncology. Vestnik Rossisko Akademii Meditsinskikh Nauk/Rossiskaia Akademiia Meditsinskikh Nauk 2003;10:34‐8. - PubMed
References to ongoing studies
AMAROS {published data only}
-
- Donker M, Tienhoven G, Straver ME, Meijnen P, Velde CJH, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981‐22023 AMAROS): a randomised, multicentre, open‐label, phase 3 non‐inferiority trial. Lancet Oncology 2014;15:1303‐10. - PMC - PubMed
GF‐GS 01 {published data only}
-
- GF‐GS 01/NCT00144898. Ongoing study 2003.
KiSS {published data only}
-
- Helms G, Kuhn T, Moser L, Remmel E, Kreienberg R. Shoulder‐arm morbidity in patients with sentinel node biopsy and complete axillary dissection ‐ data from a prospective randomised trial. European Journal of Surgical Oncology 2009;35(7):696‐701. - PubMed
-
- Schem C, Jonat W, Ostertag H. Observation or standard axillary dissection after sentinel‐node biopsy in breast cancer: final results from the German KISS study. Journal of Clinical Oncology 2011;29(15 Suppl):1012.
NCT01717131 {published data only}
-
- NCT01717131/Institut Paoli‐Calmettes. Ongoing study 2012.
NCT02167490 {published data only}
-
- Sentinel Node Vs Observation After Axillary Ultra‐souND. Ongoing study 2014.
NCT02271828 {published data only}
-
- Omitting sentinel node procedure in breast cancer patients undergoing breast conserving therapy. Ongoing study 2015.
SNAC2 {published data only}
-
- SNAC2/ACTRN12605000409673. Ongoing study 2006.
SOUND {published data only}
-
- Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast 2012;21(5):678‐81. - PubMed
Additional references
Clarke 2005
-
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005;366(9503):2087‐106. - PubMed
Craig 1998
-
- Craig PH, Silverstein MJ. Small invasive carcinomas: the role of axillary lymph node dissection. In: Bland KI, Copeland EN editor(s). The Breast, Comprehensive Management of Benign and Malignant Disease. 2nd Edition. Philadelphia: Saunders, 1998:1072‐93.
EBCTCG 1998
-
- Early Breast Cancer Trialists Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. The Lancet 1998;352:930‐42. - PubMed
Goyal 2014
-
- Goyal A. POSNOC ‐ A randomised trial of armpit (axilla) treatment for women with early stage breast cancer (ISRCTN54765244), 2014. www.isrctn.com/ISRCTN54765244 accessed 12 December 2016.
Halsted 1895
Higgins 2011
Kell 2010
-
- Kell ML, Burke JP, Barry M, Morrow M. Outcome of axillary staging in early breast cancer: a meta‐analysis. Breast Cancer Research and Treatment 2010;120:441‐7. - PubMed
Kelley 1998
-
- Kelley MC, Giuliano AE. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. In: Bland KI, Copeland EN editor(s). The Breast, Comprehensive Management of Benign and Malignant Disease. 2nd Edition. Philadelphia: Saunders, 1998:1100‐11.
Kissin 1986
-
- Kissin MW, Querci della Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. British Journal of Surgery 1986;73:580‐4. - PubMed
Lefebvre 2001
-
- Lefebvre C, Clarke M. Identifying randomised trials. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration, 2011.
Lyman 2014
-
- Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early‐stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology 2014;32(13):1365‐83. [PUBMED: 24663048] - PubMed
NICE 2009
-
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and treatment. Early and Locally Advanced Breast Cancer: Diagnosis and Treatment (CG80). London: NICE, 2009. - PubMed
RevMan [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Senofski 1991
-
- Senofski GM, Moffat FL, Davis K, Masri MM, Clark KC, Robinson DS. Total axillary lymphadenectomy in the management of breast cancer. Archives of Surgery 1991;126:1336‐42. - PubMed
Tierney 2007
UICC 1987
-
- Union Internationale Contre le Cancer. TNM classification of malignant tumours.. In: Hermanek P, Sobin LH editor(s). TNM Classification of Malignant Tumours. 4th Edition. Berlin: Springer‐Verlag, 1987:80‐89..
Walsh 1989
-
- Walsh SJ, Begg CB, Carbone PP. Cancer chemotherapy in the elderly. Seminars in Oncology 1989;16:66‐75. - PubMed
Wang 2011
-
- Wang Z, Wu LC, Chen JQ. Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: a meta‐analysis. Breast Cancer Research and Treatment 2011;129:675‐89. - PubMed
Yancik 1989
-
- Yancik R, Ries LG, Yates JW. Breast cancer in ageing women. A population based study of contrasts in stage, surgery and survival. Cancer 1989;63:976‐81. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

