A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia
- PMID: 28052413
- PMCID: PMC5363238
- DOI: 10.1002/ajh.24633
A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia
Abstract
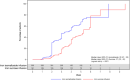
Iron deficiency anemia (IDA) is common in many chronic diseases, and intravenous (IV) iron offers a rapid and efficient iron correction. This trial compared the efficacy and safety of iron isomaltoside (also known as ferric derisomaltose) and iron sucrose in patients with IDA who were intolerant of, or unresponsive to, oral iron. The trial was an open‐label, comparative, multi‐center trial. Five hundred and eleven patients with IDA from different causes were randomized 2:1 to iron isomaltoside or iron sucrose and followed for 5 weeks. The cumulative dose of iron isomaltoside was based on body weight and hemoglobin (Hb), administered as either a 1000 mg infusion over more than 15 minutes or 500 mg injection over 2 minutes. The cumulative dose of iron sucrose was calculated according to Ganzoni and administered as repeated 200 mg infusions over 30 minutes. The mean cumulative dose of iron isomaltoside was 1640.2 (standard deviation (SD): 357.6) mg and of iron sucrose 1127.9 (SD: 343.3) mg. The primary endpoint was the proportion of patients with a Hb increase ≥2 g/dL from baseline at any time between weeks 1‐5. Both non‐inferiority and superiority were confirmed for the primary endpoint, and a shorter time to Hb increase ≥2 g/dL was observed with iron isomaltoside. For all biochemical efficacy parameters, faster and/or greater improvements were found with iron isomaltoside. Both treatments were well tolerated; 0.6% experienced a serious adverse drug reaction. Iron isomaltoside was more effective than iron sucrose in achieving a rapid improvement in Hb. Furthermore, iron isomaltoside has an advantage over iron sucrose in allowing higher cumulative dosing in fewer administrations. Both treatments were well tolerated in a broad population with IDA.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Lars L. Thomsen is employed by Pharmacosmos A/S, and the investigators/institutions received a fee per patient. Richard Derman has been a consultant for Pharmacosmos A/S. Michael Auerbach has received research funding from Pharmacosmos A/S and AMAG Pharmaceuticals and has consulted for Pharmacosmos A/S, AMAG Pharmaceuticals, and Luitpold Pharmaceuticals. Eloy Roman, Manuel R. Modiano, and Maureen M. Okam have no further conflicts of interest.
Figures
References
-
- Groopman JE, Itri LM. Chemotherapy‐induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. - PubMed
-
- Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol. 2008;52:501–511. - PubMed
-
- Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116(Suppl7A):44S–49S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources