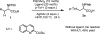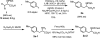Ligand-Enabled β-C-H Arylation of α-Amino Acids Without Installing Exogenous Directing Groups
- PMID: 28052530
- PMCID: PMC5302090
- DOI: 10.1002/anie.201610580
Ligand-Enabled β-C-H Arylation of α-Amino Acids Without Installing Exogenous Directing Groups
Abstract
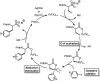
Herein we report acid-directed β-C(sp3 )-H arylation of α-amino acids enabled by pyridine-type ligands. This reaction does not require the installation of an exogenous directing group, is scalable, and enables the preparation of Fmoc-protected unnatural amino acids in three steps. The pyridine-type ligands are crucial for the development of this new C(sp3 )-H arylation.
Keywords: C−H activation; amino acids; arylation; palladium; pyridine ligands.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- For selected reviews and discussion on utility of carboxylic acids, see.
- Gooßen LJ, Rodriguez N, Gooßen K. Angew. Chem. Int. Ed. 2008;47:3100. - PubMed
- Angew. Chem. 2008;120:3144.
- Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. Science. 2016;352:801. - PMC - PubMed
-
- Kao L, Sen A. J. Chem. Soc, C.C. 1991:1242.
- Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149. - PubMed
- Lee JM, Chang S. Tetrahedron Lett. 2006;47:1375.
-
- Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunder LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510. - PubMed
-
- Novák P, Correa A, Gallardo-Donaire J, Martin R. Angew. Chem. Int. Ed. 2011;50:12236. - PubMed
- Angew.Chem. 2011;123:12444.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources