The Oral β-Lactamase SYN-004 (Ribaxamase) Degrades Ceftriaxone Excreted into the Intestine in Phase 2a Clinical Studies
- PMID: 28052855
- PMCID: PMC5328510
- DOI: 10.1128/AAC.02197-16
The Oral β-Lactamase SYN-004 (Ribaxamase) Degrades Ceftriaxone Excreted into the Intestine in Phase 2a Clinical Studies
Abstract
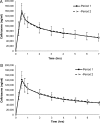
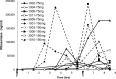
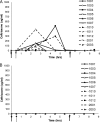
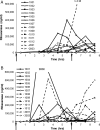
SYN-004 (ribaxamase) is a β-lactamase designed to be orally administered concurrently with intravenous β-lactam antibiotics, including most penicillins and cephalosporins. Ribaxamase's anticipated mechanism of action is to degrade excess β-lactam antibiotic that is excreted into the small intestine. This enzymatic inactivation of excreted antibiotic is expected to protect the gut microbiome from disruption and thus prevent undesirable side effects, including secondary infections such as Clostridium difficile infections, as well as other antibiotic-associated diarrheas. In phase 1 clinical studies, ribaxamase was well tolerated compared to a placebo group and displayed negligible systemic absorption. The two phase 2a clinical studies described here were performed to confirm the mechanism of action of ribaxamase, degradation of β-lactam antibiotics in the human intestine, and were therefore conducted in subjects with functioning ileostomies to allow serial sampling of their intestinal chyme. Ribaxamase fully degraded ceftriaxone to below the level of quantitation in the intestines of all subjects in both studies. Coadministration of oral ribaxamase with intravenous ceftriaxone was also well tolerated, and the plasma pharmacokinetics of ceftriaxone were unchanged by ribaxamase administration. Since ribaxamase is formulated as a pH-dependent, delayed-release formulation, the activity of ribaxamase in the presence of the proton pump inhibitor esomeprazole was examined in the second study; coadministration of these drugs did not adversely affect ribaxamase's ability to degrade ceftriaxone excreted into the intestine. These studies have confirmed the in vivo mechanism of action of ribaxamase, degradation of β-lactam antibiotics in the human intestine (registered at ClinicalTrials.gov under NCT02419001 and NCT02473640).
Keywords: beta-lactamases; ceftriaxone; clinical trials; dysbiosis; gut microbiome; oral administration; protection.
Copyright © 2017 Kokai-Kun et al.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

