Neural Variability Quenching Predicts Individual Perceptual Abilities
- PMID: 28053033
- PMCID: PMC6705669
- DOI: 10.1523/JNEUROSCI.1671-16.2016
Neural Variability Quenching Predicts Individual Perceptual Abilities
Abstract
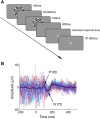
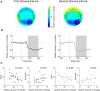
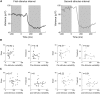
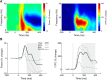
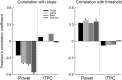
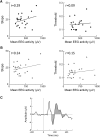
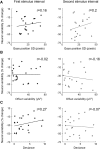
Neural activity during repeated presentations of a sensory stimulus exhibits considerable trial-by-trial variability. Previous studies have reported that trial-by-trial neural variability is reduced (quenched) by the presentation of a stimulus. However, the functional significance and behavioral relevance of variability quenching and the potential physiological mechanisms that may drive it have been studied only rarely. Here, we recorded neural activity with EEG as subjects performed a two-interval forced-choice contrast discrimination task. Trial-by-trial neural variability was quenched by ∼40% after the presentation of the stimulus relative to the variability apparent before stimulus presentation, yet there were large differences in the magnitude of variability quenching across subjects. Individual magnitudes of quenching predicted individual discrimination capabilities such that subjects who exhibited larger quenching had smaller contrast discrimination thresholds and steeper psychometric function slopes. Furthermore, the magnitude of variability quenching was strongly correlated with a reduction in broadband EEG power after stimulus presentation. Our results suggest that neural variability quenching is achieved by reducing the amplitude of broadband neural oscillations after sensory input, which yields relatively more reproducible cortical activity across trials and enables superior perceptual abilities in individuals who quench more.
Significance statement: Variability quenching is a phenomenon in which neural variability across trials is reduced by the presentation of a stimulus. Although this phenomenon has been reported across a variety of animal and human studies, its functional significance and behavioral relevance have been examined only rarely. Here, we report novel empirical evidence from humans revealing that variability quenching differs dramatically across individual subjects and explains to a certain degree why some individuals exhibit better perceptual abilities than others. In addition, we found a strong relationship between variability quenching and suppression of broadband neural oscillations. Together, our results reveal the importance of reproducible cortical activity for enabling better perceptual abilities and suggest a potential underlying mechanism that may explain why variability quenching occurs.
Keywords: EEG; trial by trial neural variability; variability quenching; visual perception.
Copyright © 2017 the authors 0270-6474/17/370097-13$15.00/0.
Figures




References
-
- Babiloni C, Vecchio F, Bultrini A, Luca Romani G, Rossini PM. Pre- and poststimulus alpha rhythms are related to conscious visual perception: a high-resolution EEG study. Cereb Cortex. 2006;16:1690–1700. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
