Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus
- PMID: 28053043
- PMCID: PMC5214632
- DOI: 10.1523/JNEUROSCI.1353-16.2016
Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus
Abstract
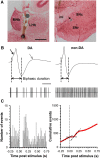
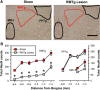
Neurons in the lateral habenula (LHb) are transiently activated by aversive events and have been implicated in associative learning. Functional changes associated with tonic and phasic activation of the LHb are often attributed to a corresponding inhibition of midbrain dopamine (DA) neurons. Activation of GABAergic neurons in the rostromedial tegmental nucleus (RMTg), a region that receives dense projections from the LHb and projects strongly to midbrain monoaminergic nuclei, is believed to underlie the transient inhibition of DA neurons attributed to activation of the LHb. To test this premise, the effects of axon-sparing lesions of the RMTg were assessed on LHb-induced inhibition of midbrain DA cell firing in anesthetized rats. Quinolinic acid lesions decreased the number of NeuN-positive neurons in the RMTg significantly while largely sparing cells in neighboring regions. Lesions of the RMTg reduced both the number of DA neurons inhibited by, and the duration of inhibition resulting from, LHb stimulation. Although the firing rate was not altered, the regularity of DA cell firing was increased in RMTg-lesioned rats. Locomotor activity in an open field was also elevated. These results are the first to show that RMTg neurons contribute directly to LHb-induced inhibition of DA cell activity and support the widely held proposition that GABAergic neurons in the mesopontine tegmentum are an important component of a pathway that enables midbrain DA neurons to encode the negative valence associated with failed expectations and aversive stimuli.
Significance statement: Phasic changes in the activity of midbrain dopamine cells motivate and guide future behavior. Activation of the lateral habenula by aversive events inhibits dopamine neurons transiently, providing a neurobiological representation of learning models that incorporate negative reward prediction errors. Anatomical evidence suggests that this inhibition occurs via the rostromedial tegmental nucleus, but this hypothesis has yet to be tested directly. Here, we show that axon-sparing lesions of the rostromedial tegmentum attenuate habenula-induced inhibition of dopamine neurons significantly. These data support a substantial role for the rostromedial tegmentum in habenula-induced feedforward inhibition of dopamine neurons.
Keywords: RMTg; firing pattern; prediction error; salience; tVTA.
Copyright © 2017 the authors 0270-6474/17/370217-09$15.00/0.
Figures



References
-
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. - DOI - PMC - PubMed
-
- Bourdy R, Sánchez-Catalán MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, Sesack SR, Georges F, Barrot M. Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology. 2014;39:2788–2798. doi: 10.1038/npp.2014.129. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
