Inactivation of Rab11a GTPase in Macrophages Facilitates Phagocytosis of Apoptotic Neutrophils
- PMID: 28053235
- PMCID: PMC5296368
- DOI: 10.4049/jimmunol.1601495
Inactivation of Rab11a GTPase in Macrophages Facilitates Phagocytosis of Apoptotic Neutrophils
Abstract
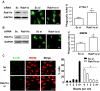
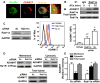
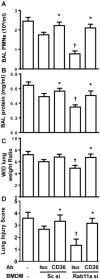
The timely and efficient clearance of apoptotic neutrophils by macrophages (efferocytosis) is required for the resolution of inflammation and tissue repair, but the regulatory mechanisms remain unclear. In this study, we investigated the role of the small GTPase Ras-related protein in brain (Rab)11a in regulating efferocytosis, and on this basis the resolution of inflammatory lung injury. We observed that apoptotic neutrophil feeding induced a rapid loss of Rab11a activity in bone marrow-derived macrophages and found that depletion of Rab11a in macrophages by small interfering RNA dramatically increased the phagocytosis of apoptotic neutrophils compared with control cells. Additionally, overexpression of wild-type Rab11a inhibited macrophage efferocytosis, whereas overexpression of dominant-negative Rab11a (Rab11a S25N) increased the clearance of apoptotic neutrophils. Rab11a knockdown also increased the surface level of CD36 in macrophages, but it reduced cell surface expression of a disintegrin and metalloproteinase (ADAM) 17. Depletion of ADAM17 rescued the decreased surface CD36 expression found in macrophages overexpressing wild-type Rab11a. Also, blockade of CD36 abolished the augmented efferocytosis seen in Rab11a-depleted macrophages. In mice challenged with endotoxin, intratracheal instillation of Rab11a-depleted macrophages reduced neutrophil count in bronchoalveolar lavage fluid, increased the number of macrophages containing apoptotic neutrophils, and prevented inflammatory lung injury. Thus, Rab11a inactivation in macrophages as a result of apoptotic cell binding initiates phagocytosis of apoptotic neutrophils via the modulation of ADAM17-mediated CD36 cell surface expression. Our results raise the possibility that inhibition of Rab11a activity in macrophages is a promising strategy for activating the resolution of inflammatory lung injury.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures







Similar articles
-
TLR4 is required for macrophage efferocytosis during resolution of ventilator-induced lung injury.Am J Physiol Lung Cell Mol Physiol. 2021 Oct 1;321(4):L787-L801. doi: 10.1152/ajplung.00226.2021. Epub 2021 Aug 18. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34405715 Free PMC article.
-
Isoflurane promotes phagocytosis of apoptotic neutrophils through AMPK-mediated ADAM17/Mer signaling.PLoS One. 2017 Jul 3;12(7):e0180213. doi: 10.1371/journal.pone.0180213. eCollection 2017. PLoS One. 2017. PMID: 28671983 Free PMC article.
-
Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype.Circ Res. 2013 Jun 21;113(1):52-61. doi: 10.1161/CIRCRESAHA.112.300683. Epub 2013 Apr 12. Circ Res. 2013. PMID: 23584255 Free PMC article.
-
Clearance of apoptotic neutrophils and resolution of inflammation.Immunol Rev. 2016 Sep;273(1):357-70. doi: 10.1111/imr.12453. Immunol Rev. 2016. PMID: 27558346 Free PMC article. Review.
-
Regulation of macrophage activation by proteins expressed on apoptotic neutrophils: Subversion towards autoimmunity by proteinase 3.Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12990. doi: 10.1111/eci.12990. Eur J Clin Invest. 2018. PMID: 30039869 Review.
Cited by
-
Endosomal trafficking of two-pore K+ efflux channel TWIK2 to plasmalemma mediates NLRP3 inflammasome activation and inflammatory injury.Elife. 2023 May 9;12:e83842. doi: 10.7554/eLife.83842. Elife. 2023. PMID: 37158595 Free PMC article.
-
Beclin-1-Dependent Autophagy Improves Outcomes of Pneumonia-Induced Sepsis.Front Cell Infect Microbiol. 2021 Jun 15;11:706637. doi: 10.3389/fcimb.2021.706637. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34211859 Free PMC article.
-
Introduction to the Community of Extracellular Vesicles.Subcell Biochem. 2021;97:3-18. doi: 10.1007/978-3-030-67171-6_1. Subcell Biochem. 2021. PMID: 33779911
-
Cellular mechanisms underlying the impairment of macrophage efferocytosis.Immunol Lett. 2023 Feb;254:41-53. doi: 10.1016/j.imlet.2023.02.001. Epub 2023 Feb 4. Immunol Lett. 2023. PMID: 36740099 Free PMC article. Review.
-
The GTPase Rab1 Is Required for NLRP3 Inflammasome Activation and Inflammatory Lung Injury.J Immunol. 2019 Jan 1;202(1):194-206. doi: 10.4049/jimmunol.1800777. Epub 2018 Nov 19. J Immunol. 2019. PMID: 30455398 Free PMC article.
References
-
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR. Immunosuppressive effect of apoptotic cells. Nature. 1997;390:350–351. - PubMed
-
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidyiserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

