Spotlight on the 9-valent HPV vaccine
- PMID: 28053505
- PMCID: PMC5191848
- DOI: 10.2147/DDDT.S91018
Spotlight on the 9-valent HPV vaccine
Abstract
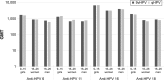
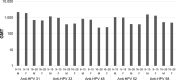
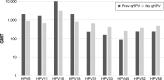
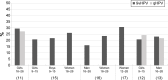
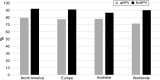
Starting in 2006, vaccination against human papillomavirus (HPV) has been progressively implemented in most developed countries. Two vaccines have been successfully used, a bivalent vaccine targeting HPV-related cancers (bHPV) and a quadrivalent vaccine (qHPV) targeting both HPV-related cancers and genital warts. Between December 2014 and June 2015, a new nonavalent HPV vaccine (9vHPV) was granted marketing authorization in the USA and Europe. The 9vHPV was developed from the qHPV and includes five additional HPV types that should increase the level of protection toward HPV-related cancers. Efficacy and/or immunogenicity of 9vHPV has been assessed in eight clinical studies. The 9vHPV vaccine induced a very robust immune response against all vaccine types, with seroconversion rates close to 100%. The safety profile of 9vHPV is comparable to that of qHPV. Local reactions, especially swelling, have been more frequently reported after 9vHPV than qHPV, and this slightly increases when the 9vHPV is coadministered with other vaccines. The additional coverage offered by the 9vHPV may prevent a significant proportion of HPV-related cancers (variable between 8% and 18%) depending on the local distribution of high-risk HPV types in the population. It is impossible, at present, to anticipate the actual impact of the wide use of the 9vHPV in comparison with the bHPV or the qHPV, since it depends on many variables including duration of protection, potential cross-protection toward nonvaccine types, and herd immunity effect.
Keywords: cervical cancer; genital warts; head and neck cancer; human papillomavirus vaccine; immunogenicity; vaccine safety.
Conflict of interest statement
The author reports no conflicts of interest in this work.
Figures
References
-
- Rigoni-Stern D. Fatti statistici relative alle mallatie cancrosi che servirono de base alla poche cose dette dal dott. [Surgical facts, related to cancer diseases] G Servire Progr Path Tera. 1842;2:507–517. Italian.
-
- Zur Hausen H, Gissmann L, Steiner W, Dippold W, Dregger I. Human papilloma viruses and cancer. Bibl Haematol. 1975;43:569–571. - PubMed
-
- Zur Hausen H. Oncogenic herpes viruses. Biochim Biophys Acta. 1974;417(1):25–53. - PubMed
-
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. - PubMed
-
- International Agency for Research on Cancer. World Health Organisation IARC Monographs on the Evaluation of the Carcinogenic Risks to Human. 2016. [Accessed June 24, 2016]. Available from: http://monographs.iarc.fr/ENG/Classification/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

