Development of a chitosan based double layer-coated tablet as a platform for colon-specific drug delivery
- PMID: 28053506
- PMCID: PMC5191854
- DOI: 10.2147/DDDT.S123412
Development of a chitosan based double layer-coated tablet as a platform for colon-specific drug delivery
Abstract
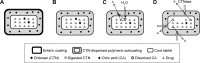
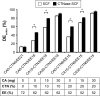
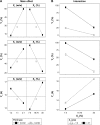
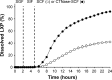
A double layer-coated colon-specific drug delivery system (DL-CDDS) was developed, which consisted of chitosan (CTN) based polymeric subcoating of the core tablet containing citric acid for microclimate acidification, followed by an enteric coating. The polymeric composition ratio of Eudragit E100 and ethyl cellulose and amount of subcoating were optimized using a two-level factorial design method. Drug-release characteristics in terms of dissolution efficiency and controlled-release duration were evaluated in various dissolution media, such as simulated colonic fluid in the presence or absence of CTNase. Microflora activation and a stepwise mechanism for drug release were postulated. Consequently, the optimized DL-CDDS showed drug release in a controlled manner by inhibiting drug release in the stomach and intestine, but releasing the drug gradually in the colon (approximately 40% at 10 hours and 92% at 24 hours in CTNase-supplemented simulated colonic fluid), indicating its feasibility as a novel platform for CDD.
Keywords: acidification; chitosan; colon-specific delivery; controlled release; factorial design; microflora.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224(1–2):19–38. - PubMed
-
- Pawar PK, Gautam C. Design, optimization and evaluation of mesalamine matrix tablet for colon drug delivery system. J Pharm Invest. 2016;46(1):67–78.
-
- Steed KP, Hooper G, Monti N, Benedetti MS, Fornasini G, Wilding IR. The use of pharmacoscintigraphy to focus the development strategy for a novel 5-ASA colon targeting system (Time Clock system) J Control Release. 1997;49(2–3):115–122.
-
- Muraoka M, Hu Z, Shimokawa T, et al. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J Control Release. 1998;52(1–2):119–129. - PubMed
-
- You YC, Dong LY, Dong K, et al. In vitro and in vivo application of pH-sensitive colon-targeting polysaccharide hydrogel used for ulcerative colitis therapy. Carbohydr Polym. 2015;130:243–253. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

