Bottom-up fabrication of zwitterionic polymer brushes on intraocular lens for improved biocompatibility
- PMID: 28053528
- PMCID: PMC5191625
- DOI: 10.2147/IJN.S107491
Bottom-up fabrication of zwitterionic polymer brushes on intraocular lens for improved biocompatibility
Abstract
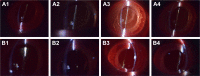
Intraocular lens (IOL) is an efficient implantable device commonly used for treating cataracts. However, bioadhesion of bacteria or residual lens epithelial cells on the IOL surface after surgery causes postoperative complications, such as endophthalmitis or posterior capsular opacification, and leads to loss of sight again. In the present study, zwitterionic polymer brushes were fabricated on the IOL surface via bottom-up grafting procedure. The attenuated total reflection-Fourier transform infrared and contact angle measurements indicated successful surface modification, as well as excellent hydrophilicity. The coating of hydrophilic zwitterionic polymer effectively decreased the bioadhesion of lens epithelial cells or bacteria. In vivo intraocular implantation results showed good in vivo biocompatibility of zwitterionic IOL and its effectiveness against postoperative complications.
Keywords: PCO; RAFT; endophthalmitis; in vivo; surface modification.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
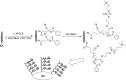






References
-
- Nibourg LM, Gelens E, Kuijer R, Hooymans JMM, van Kooten TG, Koopmans SA. Prevention of posterior capsular opacification. Exp Eye Res. 2015;136:100–115. - PubMed
-
- Saika S, Kawashima Y, Miyamoto T, et al. Pathological findings in lens capsule and silicone intraocular lens extracted from eye with chronic infectious endophthalmitis. JNP J Ophthalmol. 1999;42(6):456–460. - PubMed
-
- Okajima Y. Biofilm formation by Staphylococcus epidermidis on intraocular lens material. Invest Ophthalmol Vis Sci. 2006;47(7):2971–2975. - PubMed
-
- Lin QK, Ren KF, Ji J. Hyaluronic acid and chitosan-DNA complex multilayered thin film as surface-mediated nonviral gene delivery system. Colloids Surf B Biointerfaces. 2009;74(1):298–303. - PubMed
-
- Lin QK, Ding X, Qiu FY, Song XX, Fu GS, Ji J. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayer. Biomaterials. 2010;31(14):4017–4025. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

