Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer
- PMID: 28053592
- PMCID: PMC5198976
- DOI: 10.1016/j.sjbs.2016.02.025
Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer
Abstract
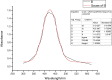
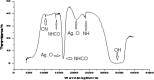
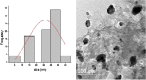
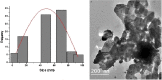
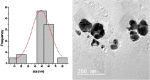
Synthesis of silver nanoparticles (AgNPs) has become a necessary field of applied science. Biological method for synthesis of AgNPs by Rhizopus stolonifer aqueous mycelial extract was used. The AgNPs were identified by UV-visible spectrometry, X-ray diffraction (XRD), transmission electron microscopy (TEM) and Fourier transform infrared spectrometry (FT-IR). The presence of surface plasmon band around 420 nm indicates AgNPs formation. The characteristic of the AgNPs within the face-centered cubic (fcc) structure are indicated by the peaks of the X-ray diffraction (XRD) pattern corresponding to (1 1 1), (2 0 0) and (2 2 0) planes. Spherical, mono-dispersed and stable AgNPs with diameter around 9.47 nm were prepared and affirmed by high-resolution transmission electron microscopy (HR-TEM). Fourier Transform Infrared (FTIR) shows peaks at 1426 and 1684 cm-1 that affirm the presence of coat covering protein the AgNPs which is known as capping proteins. Parameter optimization showed the smallest size of AgNPs (2.86 ± 0.3 nm) was obtained with 10-2 M AgNO3 at 40 °C. The present study provides the proof that the molecules within aqueous mycelial extract of R. stolonifer facilitate synthesis of AgNPs and highlight on value-added from R. stolonifer for cost effectiveness. Also, eco-friendly medical and nanotechnology-based industries could also be provided. Size of prepared AgNPs could be controlled by temperature and AgNO3 concentration. Further studies are required to study effect of more parameters on size and morphology of AgNPs as this will help in the control of large scale production of biogenic AgNPs.
Keywords: Nanoparticles; Rhizopus stolonifer; Silver.
Figures






References
-
- Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B. 2003;28:313–318.
-
- Balaji D., Basavaraja S., Deshpande R., Bedre M., Prabhakara B., Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B. 2009;68:88–92. - PubMed
-
- Balali G.R., Neate S.M., Scott E.S., Whisson D.L., Wicks T.J. Anastomosis group and pathogenicity of isolates of Rhizoctonia solani from potato crops in South Australia. Plant Pathol. 1995;44(6):1050–1057.
-
- Basavaraj U., Praveenkumar N., Sabiha T.S., Rupali S., Samprita B. Synthesis and characterization of silver nanoparticles. Int. J. Pharm. Bio Sci. 2012;3:10–14.
-
- Basavaraja S., Balaji S., Lagashetty K., Rajasab H., Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008;43:1164–1170.
LinkOut - more resources
Full Text Sources
Other Literature Sources

