Fibrinogen and Fibronectin Binding Activity and Immunogenic Nature of Choline Binding Protein M
- PMID: 28053927
- PMCID: PMC5207102
Fibrinogen and Fibronectin Binding Activity and Immunogenic Nature of Choline Binding Protein M
Abstract
Background: Choline-binding proteins (CBPs) are a group of surface-exposed proteins, which play crucial and physiological roles in Streptococcus pneumoniae. The novel member of CBPs, choline-binding protein M (CbpM) may have binding activity to plasma proteins. This study aimed to clone and express CbpM and demonstrate its interaction with plasma proteins and patients' sera.
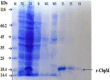
Methods: The total length of cbpM gene was cloned in pET21a vector and expressed in BL21 expression host. Verification of recombinant protein was evaluated by Western blot using anti-His tag monoclonal antibody. Binding ability of the recombinant protein to plasma proteins and the interaction with patients' sera were assessed by Western blot and ELISA methods.
Results: The cbpM gene was successfully cloned into pET21a and expressed in BL21 host. Binding activity to fibronectin and fibrinogen and antibody reaction of CbpM to patients' sera was demonstrated by Western blot and ELISA methods, respectively.
Conclusion: CbpM is one of the pneumococcal surface-exposed proteins, which mediates pneumococcal binding to fibronectin and fibrinogen proteins.
Keywords: Choline-binding protein M; Fibrinogen; Fibronectin; Streptococcus pneumoniae.
Figures






References
-
- Feldman C, Anderson R. (2014). Review: current and new generation pneumococcal vaccines. J Infect, 69 ( 4 ): 309 –25. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
