Guanidinium can both Cause and Prevent the Hydrophobic Collapse of Biomacromolecules
- PMID: 28054487
- PMCID: PMC5499822
- DOI: 10.1021/jacs.6b11082
Guanidinium can both Cause and Prevent the Hydrophobic Collapse of Biomacromolecules
Abstract
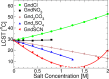
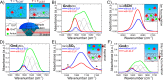
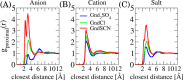
A combination of Fourier transform infrared and phase transition measurements as well as molecular computer simulations, and thermodynamic modeling were performed to probe the mechanisms by which guanidinium (Gnd+) salts influence the stability of the collapsed versus uncollapsed state of an elastin-like polypeptide (ELP), an uncharged thermoresponsive polymer. We found that the cation's action was highly dependent upon the counteranion with which it was paired. Specifically, Gnd+ was depleted from the ELP/water interface and was found to stabilize the collapsed state of the macromolecule when paired with well-hydrated anions such as SO42-. Stabilization in this case occurred via an excluded volume (or depletion) effect, whereby SO42- was strongly partitioned away from the ELP/water interface. Intriguingly, at low salt concentrations, Gnd+ was also found to stabilize the collapsed state of the ELP when paired with SCN-, which is a strong binder for the ELP. In this case, the anion and cation were both found to be enriched in the collapsed state of the polymer. The collapsed state was favored because the Gnd+ cross-linked the polymer chains together. Moreover, the anion helped partition Gnd+ to the polymer surface. At higher salt concentrations (>1.5 M), GndSCN switched to stabilizing the uncollapsed state because a sufficient amount of Gnd+ and SCN- partitioned to the polymer surface to prevent cross-linking from occurring. Finally, in a third case, it was found that salts which interacted in an intermediate fashion with the polymer (e.g., GndCl) favored the uncollapsed conformation at all salt concentrations. These results provide a detailed, molecular-level, mechanistic picture of how Gnd+ influences the stability of polypeptides in three distinct physical regimes by varying the anion. It also helps explain the circumstances under which guanidinium salts can act as powerful and versatile protein denaturants.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Kunz W.; Lo Nostro P.; Ninham B. W. The Present State of Affairs with Hofmeister Effects. Curr. Opin. Colloid Interface Sci. 2004, 9, 1–18. 10.1016/j.cocis.2004.05.004. - DOI
-
- Leontidis E. Hofmeister Anion Effects on Surfactant Self-Assembly and the Formation of Mesoporous Solids. Curr. Opin. Colloid Interface Sci. 2002, 7, 81–91. 10.1016/S1359-0294(02)00010-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

