RNY (YRNA)-derived small RNAs regulate cell death and inflammation in monocytes/macrophages
- PMID: 28055017
- PMCID: PMC5386355
- DOI: 10.1038/cddis.2016.429
RNY (YRNA)-derived small RNAs regulate cell death and inflammation in monocytes/macrophages
Abstract
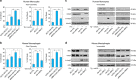
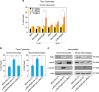
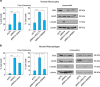
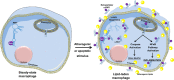
The recent discovery of new classes of small RNAs has opened unknown territories to explore new regulations of physiopathological events. We have recently demonstrated that RNY (or Y RNA)-derived small RNAs (referred to as s-RNYs) are an independent class of clinical biomarkers to detect coronary artery lesions and are associated with atherosclerosis burden. Here, we have studied the role of s-RNYs in human and mouse monocytes/macrophages and have shown that in lipid-laden monocytes/macrophages s-RNY expression is timely correlated to the activation of both NF-κB and caspase 3-dependent cell death pathways. Loss- or gain-of-function experiments demonstrated that s-RNYs activate caspase 3 and NF-κB signaling pathways ultimately promoting cell death and inflammatory responses. As, in atherosclerosis, Ro60-associated s-RNYs generated by apoptotic macrophages are released in the blood of patients, we have investigated the extracellular function of the s-RNY/Ro60 complex. Our data demonstrated that s-RNY/Ro60 complex induces caspase 3-dependent cell death and NF-κB-dependent inflammation, when added to the medium of cultured monocytes/macrophages. Finally, we have shown that s-RNY function is mediated by Toll-like receptor 7 (TLR7). Indeed using chloroquine, which disrupts signaling of endosome-localized TLRs 3, 7, 8 and 9 or the more specific TLR7/9 antagonist, the phosphorothioated oligonucleotide IRS954, we blocked the effect of either intracellular or extracellular s-RNYs. These results position s-RNYs as relevant novel functional molecules that impacts on macrophage physiopathology, indicating their potential role as mediators of inflammatory diseases, such as atherosclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

