SERS-enhanced piezoplasmonic graphene composite for biological and structural strain mapping
- PMID: 28055038
- PMCID: PMC5266539
- DOI: 10.1039/c6nr09005b
SERS-enhanced piezoplasmonic graphene composite for biological and structural strain mapping
Abstract
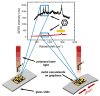
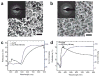
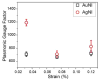
Thin-film optical strain sensors have the ability to map small deformations with spatial and temporal resolution and do not require electrical interrogation. This paper describes the use of graphene decorated with metallic nanoislands for sensing of tensile deformations of less than 0.04% with a resolution of less than 0.002%. The nanoisland-graphene composite films contain gaps between the nanoislands, which when functionalized with benzenethiolate behave as hot spots for surface-enhanced Raman scattering (SERS). Mechanical strain increases the sizes of the gaps; this increase attenuates the electric field, and thus attenuates the SERS signal. This compounded, SERS-enhanced "piezoplasmonic" effect can be quantified using a plasmonic gauge factor, and is among the most sensitive mechanical sensors of any type. Since the graphene-nanoisland films are both conductive and optically active, they permit simultaneous electrical stimulation of myoblast cells and optical detection of the strains produced by the cellular contractions.
Figures



References
-
- Li M, Tang HX, Roukes ML. Nat Nanotechnol. 2007;2:114. - PubMed
-
- Liu X, Mwangi M, Li X, O’Brien M, Whitesides GM. Lab on a Chip. 2011;11:2189. - PubMed
-
- Pacelli M, Caldani L, Paradiso R. Annu. Int. Conf. IEEE Eng. Med. Biol. Proc; 2006; p. 5358. - PubMed
-
- Mason WP, Thurston RN. J Acoust Soc Am. 1957;29:1096.
-
- Segev-Bar M, Landman A, Nir-Shapira M, Shuster G, Haick H. ACS Appl Mater Interfaces. 2013;5:5531. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

