Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight
- PMID: 28055105
- PMCID: PMC5610154
- DOI: 10.1111/bph.13703
Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight
Abstract
Background and purpose: We have recently shown that a reduced function of endothelial nitric oxide synthase (eNOS) in the perivascular adipose tissue (PVAT) contributes crucially to obesity-induced vascular dysfunction in mice. The current study was conducted to test the hypothesis that vascular dysfunction in obesity can be reversed by in vivo improvement of PVAT eNOS activity.
Experimental approach: Male C57BL/6J mice were fed a high-fat diet (HFD) for 22 weeks to induce obesity. During the last 4 weeks of HFD feeding, the obese mice were treated p.o. with the standardized Crataegus extract WS® 1442, which has been shown previously to improve eNOS activity.
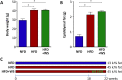
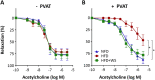
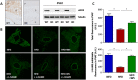
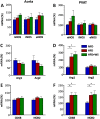
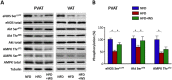
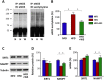
Key results: Diet-induced obesity in mice markedly reduced the vasodilator response of thoracic aorta to acetylcholine in wire myograph experiments. Strikingly, this vascular dysfunction was only evident in PVAT-containing aorta but not in PVAT-free aorta. In vivo treatment of obese mice with WS® 1442 had no effect on body weight or epididymal fat mass, but completely restored the vascular function of PVAT-containing aorta. Feeding a HFD led to a reduced phosphorylation and an enhanced acetylation of PVAT eNOS, both effects were reversed by WS® 1442 treatment.
Conclusion and implications: PVAT plays a key role in vascular dysfunction in diet-induced obese mice. Not obesity itself, but a PVAT dysfunction is responsible for obesity-induced vascular disorders. Improving PVAT function by pharmacological means (e.g. with WS® 1442) can ameliorate vascular function even without reducing body weight or fat mass.
Linked articles: This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue - Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc.
© 2017 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures
Similar articles
-
Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice.Br J Pharmacol. 2017 Oct;174(20):3527-3541. doi: 10.1111/bph.13687. Epub 2017 Jan 12. Br J Pharmacol. 2017. PMID: 27930804 Free PMC article.
-
Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):78-85. doi: 10.1161/ATVBAHA.115.306263. Epub 2015 Nov 19. Arterioscler Thromb Vasc Biol. 2016. PMID: 26586660
-
The role of perivascular adipose tissue in obesity-induced vascular dysfunction.Br J Pharmacol. 2017 Oct;174(20):3425-3442. doi: 10.1111/bph.13650. Epub 2016 Nov 17. Br J Pharmacol. 2017. PMID: 27761903 Free PMC article. Review.
-
Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue.Br J Pharmacol. 2017 Oct;174(20):3514-3526. doi: 10.1111/bph.13756. Epub 2017 Apr 7. Br J Pharmacol. 2017. PMID: 28236429 Free PMC article.
-
Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone.Br J Pharmacol. 2017 Oct;174(20):3388-3397. doi: 10.1111/bph.13648. Epub 2016 Nov 18. Br J Pharmacol. 2017. PMID: 27747871 Free PMC article. Review.
Cited by
-
Niacin protects against abdominal aortic aneurysm formation via GPR109A independent mechanisms: role of NAD+/nicotinamide.Cardiovasc Res. 2020 Dec 1;116(14):2226-2238. doi: 10.1093/cvr/cvz303. Cardiovasc Res. 2020. PMID: 31710686 Free PMC article.
-
Fetal programming effects of pentaerythritol tetranitrate in a rat model of superimposed preeclampsia.J Mol Med (Berl). 2020 Sep;98(9):1287-1299. doi: 10.1007/s00109-020-01949-0. Epub 2020 Aug 3. J Mol Med (Berl). 2020. PMID: 32748067 Free PMC article.
-
Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells.Arterioscler Thromb Vasc Biol. 2020 May;40(5):1094-1109. doi: 10.1161/ATVBAHA.120.312464. Epub 2020 Mar 19. Arterioscler Thromb Vasc Biol. 2020. PMID: 32188271 Free PMC article. Review.
-
Nitric oxide signalling in cardiovascular health and disease.Nat Rev Cardiol. 2018 May;15(5):292-316. doi: 10.1038/nrcardio.2017.224. Epub 2018 Feb 1. Nat Rev Cardiol. 2018. PMID: 29388567 Review.
-
Caralluma fimbriata Extract Improves Vascular Dysfunction in Obese Mice Fed a High-Fat Diet.Nutrients. 2024 Dec 12;16(24):4296. doi: 10.3390/nu16244296. Nutrients. 2024. PMID: 39770917 Free PMC article.
References
-
- Anselm E, Socorro VF, Dal‐Ros S, Schott C, Bronner C, Schini‐Kerth VB (2009). Crataegus special extract WS 1442 causes endothelium‐dependent relaxation via a redox‐sensitive Src‐ and Akt‐dependent activation of endothelial NO synthase but not via activation of estrogen receptors. J Cardiovasc Pharmacol 53: 253–260. - PubMed
-
- Bubik MF, Willer EA, Bihari P, Jurgenliemk G, Ammer H, Krombach F et al. (2012). A novel approach to prevent endothelial hyperpermeability: the Crataegus extract WS(R) 1442 targets the cAMP/Rap1 pathway. J Mol Cell Cardiol 52: 196–205. - PubMed
-
- Bussey CE, Withers SB, Aldous RG, Edwards G, Heagerty AM (2016). Obesity‐related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol 36: 1377–1385. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

