Heme and iron induce protein aggregation
- PMID: 28055290
- PMCID: PMC5361590
- DOI: 10.1080/15548627.2016.1271515
Heme and iron induce protein aggregation
Abstract
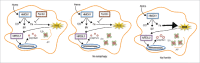
Heme is an essential molecule expressed in many tissues where it plays key roles as the prosthetic group of several proteins involved in vital physiological and metabolic processes such as gas and electron transport. Structurally, heme is a tetrapyrrole ring containing an atom of iron (Fe) in its center. When released into the extracellular milieu, heme exerts several deleterious effects, which make it an important player in infectious and noninfectious hemolytic diseases where large amounts of free heme are observed such as malaria, dengue fever, β-thalassemia, sickle cell disease and ischemia-reperfusion. Our recent work has uncovered an unappreciated cellular response triggered by heme or Fe, one of its degradation products, on macrophages, which is the formation of protein aggregates known as aggresome-like induced structres (ALIS). This response was shown to be fully dependent on ROS production and the activation of the transcription factor NFE2L2/NRF2. In addition, we have demonstrated that heme degradation by HMOX1/HO-1 (heme oxygenase 1) is required and that Fe is essential for the formation of ALIS, as heme analogs lacking the central atom of Fe are not able to induce these structures. ALIS formation is also observed in vivo, in a model of phenylhydrazine (PHZ)-induced hemolysis, indicating that it is an integral part of the host response to excessive free heme and that it may play a role in cellular homeostasis.
Keywords: ALIS; NRF2; aggregation; autophagy; ferritin; heme; iron; oxidative stress; protein.
Figures
Comment on
-
Protein aggregation as a cellular response to oxidative stress induced by heme and iron.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7474-E7482. doi: 10.1073/pnas.1608928113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821769 Free PMC article.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
