Metuzumab enhanced chemosensitivity and apoptosis in non-small cell lung carcinoma
- PMID: 28055291
- PMCID: PMC5323017
- DOI: 10.1080/15384047.2016.1276126
Metuzumab enhanced chemosensitivity and apoptosis in non-small cell lung carcinoma
Abstract
Targeted therapeutics is used as an alternative treatment of non-small cell lung cancer (NSCLC); however, treatment effect is far from being satisfactory, and therefore identification of new targets is needed. We have previously shown that metuzumab inhibit tumor growth in vivo. The present study was performed to investigate the anti-tumor efficacy of metuzumab combined with gemcitabine and cisplatin (GP), paclitaxel and cisplatin (TP) or navelbine and cisplatin (NP) regimens in multiple NSCLC cell lines. Our results demonstrate that, in comparison to single agent metuzumab or GP treated cells, metuzumab combined with GP display inhibitory effects on tumor growth. Furthermore, we found that metuzumab elevated the sensitivity of cell lines to gemcitabine, which was identified by MTT assay. Flow cytometric analysis showed that metuzumab combined with gemcitabine (GEM) treatment led to an obvious G1 arrest and an elevated apoptosis in A549, NCI-H460 and NCI-H520 cells. Western blot analysis also demonstrated a significantly reduced level of cyclin D1, Bcl-2, and an obviously increase level of Bax and full-length caspase-3 in A549, NCI-H460 and NCI-H520 cells treated with metuzumab/gemcitabine combination in comparison with single agent treated cells. In addition, metuzumab/gemcitabine treated A549, NCI-H460 and NCI-H520 cells also demonstrated a significantly increase in deoxycytidine kinase (dCK) protein level compared with single agent metuzumab or gemcitabine treated cells. Xenograft models also demonstrated that this metuzumab/gemcitabine combination led to upregulation of dCK. Taken together, the mechanisms of metuzumab combined with GP repress tumor growth were that the combined treatment significantly inhibited the tumor cell proliferation, apoptosis and cell cycle in vitro and in vivo and at least partially by induction of dCK expression. Our results suggested that metuzumab could significantly enhance chemosensitivity of human NSCLC cells to gemcitabine. Metuzumab/gemcitabine combination treatment may be a potentially useful therapeutic regimen for NSCLC patients.
Keywords: CD147; chemosensitivity; gemcitabine; metuzumab; monoclonal antibody; non-small-cell lung cancer; targeted therapy.
Figures

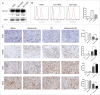


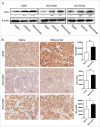
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer J Clinicians 2013; 63(1):11-30; PMID:23335087; http://dx.doi.org/10.3322/caac.21166 - DOI - PubMed
-
- Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet 2011; 378(9804):1727-1740; http://dx.doi.org/10.1016/s0140-6736(10)62101-0 - DOI - PubMed
-
- Spaks A, Svirina D, Spaka I, Jaunalksne I, Breiva D, Tracums I, Krievins D. CXC chemokine ligand 4 (CXCL4) is predictor of tumour angiogenic activity and prognostic biomarker in non-small cell lung cancer (NSCLC) patients undergoing surgical treatment. Biomarkers 2016; 21(5):474-8:1-4; PMID:27098116; http://dx.doi.org/10.3109/1354750X.2016.1172111 - DOI - PubMed
-
- Stewart RL, Carpenter BL, West DS, Knifley T, Liu L, Wang C, Weiss HL, Gal TS, Durbin EB, Arnold SM, et al.. S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide. Oncotarget 2016; 7(23):34630-42; PMID:27127879; http://dx.doi.org/10.18632/oncotarget.8969 - DOI - PMC - PubMed
-
- Xiao HQ, Tian RH, Zhang ZH, Du KQ, Ni YM. Efficacy of pemetrexed plus platinum doublet chemotherapy as first-line treatment for advanced nonsquamous non-small-cell-lung cancer: a systematic review and meta-analysis. OncoTargets therapy 2016; 9:1471-1476; PMID:27042115; http://dx.doi.org/10.2147/OTT.S96160 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
