Vascular Endothelial Over-Expression of Human Soluble Epoxide Hydrolase (Tie2-sEH Tr) Attenuates Coronary Reactive Hyperemia in Mice: Role of Oxylipins and ω-Hydroxylases
- PMID: 28056085
- PMCID: PMC5215949
- DOI: 10.1371/journal.pone.0169584
Vascular Endothelial Over-Expression of Human Soluble Epoxide Hydrolase (Tie2-sEH Tr) Attenuates Coronary Reactive Hyperemia in Mice: Role of Oxylipins and ω-Hydroxylases
Abstract
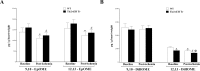
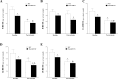
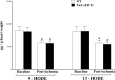
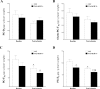
Cytochromes P450 metabolize arachidonic acid (AA) into two vasoactive oxylipins with opposing biologic effects: epoxyeicosatrienoic acids (EETs) and omega-(ω)-terminal hydroxyeicosatetraenoic acids (HETEs). EETs have numerous beneficial physiological effects, including vasodilation and protection against ischemia/reperfusion injury, whereas ω-terminal HETEs induce vasoconstriction and vascular dysfunction. We evaluated the effect of these oxylipins on post-ischemic vasodilation known as coronary reactive hyperemia (CRH). CRH prevents the potential harm associated with transient ischemia. The beneficial effects of EETs are reduced after their hydrolysis to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH). ω-terminal HETEs are formed by ω-hydroxylase family members. The relationship among endothelial over-expression of sEH (Tie2-sEH Tr), the changes in oxylipins it may produce, the pharmacologic inhibition of ω-hydroxylases, activation of PPARγ, and CRH response to a brief ischemia is not known. We hypothesized that CRH is attenuated in isolated mouse hearts with endothelial sEH over-expression through modulation of oxylipin profiles, whereas both inhibition of ω-hydroxylases and activation of PPARγ enhance CRH. Compared to WT mice, Tie2-sEH Tr mice had decreased CRH, including repayment volume, repayment duration, and repayment/debt ratio (P < 0.05), whereas inhibition of ω-hydroxylases increased these same CRH parameters in Tie2-sEH Tr mice. Inhibition of sEH with t-AUCB reversed the decreased CRH in Tie2-sEH Tr mice. Endothelial over-expression of sEH significantly changed oxylipin profiles, including decreases in DHETs, mid-chain HETEs, and prostaglandins (P < 0.05). Treatment with rosiglitazone, PPARγ-agonist, enhanced CRH (P < 0.05) in both Tie2-sEH Tr and wild type (WT) mice. These data demonstrate that endothelial over-expression of sEH (through changing the oxylipin profiles) attenuates CRH, whereas inhibition of ω-hydroxylases and activation of PPARγ enhance it.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78(3):415–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

