Nitric oxide for respiratory failure in infants born at or near term
- PMID: 28056166
- PMCID: PMC6464941
- DOI: 10.1002/14651858.CD000399.pub3
Nitric oxide for respiratory failure in infants born at or near term
Abstract
Background: Nitric oxide (NO) is a major endogenous regulator of vascular tone. Inhaled nitric oxide (iNO) gas has been investigated as treatment for persistent pulmonary hypertension of the newborn.
Objectives: To determine whether treatment of hypoxaemic term and near-term newborn infants with iNO improves oxygenation and reduces rate of death and use of extracorporeal membrane oxygenation (ECMO), or affects long-term neurodevelopmental outcomes.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), MEDLINE via PubMed (1966 to January 2016), Embase (1980 to January 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to January 2016). We searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials. We contacted the principal investigators of studies published as abstracts to ascertain the necessary information.
Selection criteria: Randomised studies of iNO in term and near-term infants with hypoxic respiratory failure, with clinically relevant outcomes, including death, use of ECMO and oxygenation.
Data collection and analysis: We analysed trial reports to assess methodological quality using the criteria of the Cochrane Neonatal Review Group. We tabulated mortality, oxygenation, short-term clinical outcomes (particularly use of ECMO) and long-term developmental outcomes.
Statistics: For categorical outcomes, we calculated typical estimates for risk ratios and risk differences. For continuous variables, we calculated typical estimates for weighted mean differences. We used 95% confidence intervals and assumed a fixed-effect model for meta-analysis.
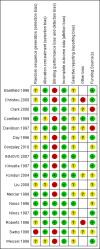
Main results: We found 17 eligible randomised controlled studies that included term and near-term infants with hypoxia.Ten trials compared iNO versus control (placebo or standard care without iNO) in infants with moderate or severe severity of illness scores (Ninos 1996; Roberts 1996; Wessel 1996; Davidson 1997; Ninos 1997; Mercier 1998; Christou 2000; Clark 2000; INNOVO 2007; Liu 2008). Mercier 1998 compared iNO versus control but allowed back-up treatment with iNO for infants who continued to satisfy the same criteria for severity of illness after two hours. This trial enrolled both preterm and term infants but reported most results separately for the two groups. Ninos 1997 studied only infants with congenital diaphragmatic hernia.One trial compared iNO versus high-frequency ventilation (Kinsella 1997).Six trials enrolled infants with moderate severity of illness scores (oxygenation index (OI) or alveolar-arterial oxygen difference (A-aDO2)) and randomised them to immediate iNO treatment or iNO treatment only after deterioration to more severe criteria (Barefield 1996; Day 1996; Sadiq 1998; Cornfield 1999; Konduri 2004; Gonzalez 2010).Inhaled nitric oxide appears to have improved outcomes in hypoxaemic term and near-term infants by reducing the incidence of the combined endpoint of death or use of ECMO (high-quality evidence). This reduction was due to a reduction in use of ECMO (with number needed to treat for an additional beneficial outcome (NNTB) of 5.3); mortality was not affected. Oxygenation was improved in approximately 50% of infants receiving iNO. The OI was decreased by a (weighted) mean of 15.1 within 30 to 60 minutes after the start of therapy, and partial pressure of arterial oxygen (PaO2) was increased by a mean of 53 mmHg. Whether infants had clear echocardiographic evidence of persistent pulmonary hypertension of the newborn (PPHN) did not appear to affect response to iNO. Outcomes of infants with diaphragmatic hernia were not improved; outcomes were slightly, but not significantly, worse with iNO (moderate-quality evidence).Infants who received iNO at less severe criteria did not have better clinical outcomes than those who were enrolled but received treatment only if their condition deteriorated. Fewer of the babies who received iNO early satisfied late treatment criteria, showing that earlier iNO reduced progression of the disease but did not further decrease mortality nor the need for ECMO (moderate-quality evidence). Incidence of disability, incidence of deafness and infant development scores were all similar between tested survivors who received iNO and those who did not.
Authors' conclusions: Inhaled nitric oxide is effective at an initial concentration of 20 ppm for term and near-term infants with hypoxic respiratory failure who do not have a diaphragmatic hernia.
Conflict of interest statement
Dr Barrington was chair of the iNO Therapeutics Canadian Medical Advisory Committee for a single meeting, for which he received expenses and an honorarium. Dr Finer previously served as a member of the iNO Therapeutics Advisory Committee.
Figures
Update of
-
Nitric oxide for respiratory failure in infants born at or near term.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD000399. doi: 10.1002/14651858.CD000399.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Jan 05;1:CD000399. doi: 10.1002/14651858.CD000399.pub3. PMID: 17054129 Updated.
References
References to studies included in this review
Barefield 1996 {published data only}
-
- Barefield ES, Karke VA, Phillips JB, Carlo WA. Inhaled nitric oxide in term infants with hypoxemic respiratory failure. Journal of Pediatrics 1996;129:279‐86. - PubMed
-
- Barefield ES, Karke VA, Phillips JB, Carlo WA. Randomized, controlled trial of inhaled NO in ECMO candidates. Pediatric Research 1995;37:195A.
Christou 2000 {published data only}
-
- Christou H, Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE, et al. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Critical Care Medicine 2000;28:3722‐7. - PubMed
Clark 2000 {published data only}
-
- Clark RH, Keuser TJ, Walker MW, Southgate MS, Huckaby JL, Perez JA, et al. for the Clinical Inhaled Nitric Oxide Research Group. Low‐dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. New England Journal of Medicine 2000;342:469‐74. - PubMed
Cornfield 1999 {published data only}
-
- Cornfield DN, Maynard RC, deRegnier RA, Guiang SF 3rd, Barbato JE, Milla CE. Randomized, controlled trial of low‐dose inhaled nitric oxide in the treatment of term and near‐term infants with respiratory failure and pulmonary hypertension. Pediatrics 1999;104:1089‐94. - PubMed
Davidson 1997 {published data only}
-
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double‐masked, placebo‐controlled, dose‐response, multicenter study. Pediatrics 1998;101:325‐34. - PubMed
-
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. I‐NO/PPHN Study Group. A double masked, randomized, placebo controlled, dose response study of inhaled nitric oxide for the treatment of persistent pulmonary hypertension of the newborn. Pediatric Research 1997;41:144A (abstract). - PubMed
Day 1996 {published data only}
-
- Day RW, Lynch JM. Acute hemodynamic and blood gas effects of inhaled nitric oxide in newborns with lung disease and pulmonary hypertension. Pediatric Research 1996;39:330A.
-
- Day RW, Lynch JM, White KS, Ward RM. Acute response to inhaled nitric oxide in newborns with respiratory failure and pulmonary hypertension. Pediatrics 1996;98:698‐705. - PubMed
Gonzalez 2010 {published data only}
-
- Gonzalez A, Fabres J, D'Apremont I, Urcelay G, Avaca M, Gandolfi C, et al. Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension. Journal of Perinatology 2010;30(6):420‐4. - PubMed
INNOVO 2007 {published data only}
-
- Field D, Elbourne D, Hardy P, Fenton AC, Ahluwalia J, Halliday HL, et al. INNOVO Trial Collaborating Group. Neonatal ventilation with inhaled nitric oxide vs. ventilatory support without inhaled nitric oxide for infants with severe respiratory failure born at or near term: the INNOVO multicentre randomised controlled trial. Neonatology 2007;91(2):73‐82. - PubMed
Kinsella 1997 {published data only}
-
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Clark RH, et al. Randomized, multicenter trial of inhaled nitric oxide and high frequency ventilation in severe persistent pulmonary hypertension of the newborn. Pediatric Research 1996;39:222A. - PubMed
-
- Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari EE, Mayock DE, et al. Randomized, multicenter trial of inhaled nitric oxide and high‐frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. Journal of Pediatrics 1997;131:55‐62. - PubMed
Konduri 2004 {published data only}
-
- Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, et al. Neonatal Inhaled Nitric Oxide Study Group. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near‐term newborn infants with hypoxic respiratory failure. Pediatrics 2004;113(3 Pt 1):559‐64. - PubMed
Liu 2008 {published data only}
-
- Liu CQ, Ma L, Tang LM, He XJ, Wei SF, Wang SX, et al. A randomized controlled study on the efficacy of inhaled nitric oxide in treatment of neonates with meconium aspiration syndrome. Zhonghua Er Ke za Zhi [Chinese Journal of Pediatrics] 2008;46(3):224‐8. - PubMed
Mercier 1998 {published data only}
-
- Franco‐Belgium Collaborative NO Trial Group. Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. Lancet 1999;354:1066‐71. - PubMed
-
- Mercier JC, Dehan M, Breart G, Clement S, O'Nody P. Inhaled nitric oxide in neonatal respiratory failure. Pediatric Research 1998;43:290A.
Ninos 1996 {published and unpublished data}
-
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full‐term and nearly full term infants with hypoxic respiratory failure. New England Journal of Medicine 1997;336:597‐604. - PubMed
-
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near‐term infants: neurodevelopmental follow‐up of the The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Journal of Pediatrics 2000;136:611‐7. - PubMed
Ninos 1997 {unpublished data only}
-
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics 1997;99:838‐45. - PubMed
-
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near‐term infants: neurodevelopmental follow‐up of the The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Journal of Pediatrics 2000;136:611‐7. - PubMed
Roberts 1996 {published data only}
-
- Roberts JD Jr, Fineman J, Morin FC III, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. New England Journal of Medicine 1997;336:605‐10. - PubMed
-
- Roberts JD Jr, Fineman J, Morin FC III, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide gas improves oxygenation in PPHN. Pediatric Research 1996;39:241A.
Sadiq 1998 {published and unpublished data}
-
- Sadiq FH, Illinois Multicenter Trial. Treatment of persistent pulmonary hypertension of the newborn with nitric oxide: a randomized trial. Pediatric Research 1998;43:192A.
-
- Sadiq HF, Mantych G, Benawra RS, Devaskar UP, Hocker JR. Inhaled nitric oxide in the treatment of moderate persistent pulmonary hypertension of the newborn: a randomized controlled, multicenter trial. Journal of Perinatology 2003;23:98‐103. - PubMed
Wessel 1996 {published data only}
-
- Ellington M, O'Reilly D, Allred EN, McCormick MC, Wessel DL, Kourembanas S. Child health status, neurodevelopmental outcome, and parental satisfaction in a randomized controlled trial of nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 2001;107:1351‐6. - PubMed
-
- Wessel D, Adatia I, Thompson J, Kane J, Marter L, Stark A, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for PPHN. Pediatric Research 1996;39:252A. - PubMed
-
- Wessel DL, Adatia I, Marter LJ, Thompson JE, Kane JW, Stark AR, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1997;100(5):e7. - PubMed
References to studies excluded from this review
Hoffman 1997 {published data only}
-
- Hoffman GM, Ross GA, Day SE, Rice TB, Nelin LD. Inhaled nitric oxide reduces the utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Critical Care Medicine 1997;25:352‐9. - PubMed
Pinheiro 1998 {published data only}
-
- Pinheiro JMB, Carey T. Inhaled NO vs. intravenous nitroprusside in neonatal pulmonary hypertension: randomized trial at a center without ECMO. Pediatric Research 1998;43:294A.
Additional references
Abman 1990
-
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium‐derived relaxing factor during transition of pulmonary circulation at birth. American Journal of Physiology 1990;259:H1921‐7. - PubMed
Beckman 1990
Bouchet 1993
-
- Bouchet M, Renaudin MH, Raveau C, Mercier JC Dehan M, Zupan V. Safety requirement for use of inhaled nitric oxide in neonates. Lancet 1993;341:968‐9. - PubMed
Cornfield 1992
-
- Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH. Effects of birth‐related stimuli on L‐arginine‐dependent pulmonary vasodilation in ovine fetus. American Journal of Physiology 1992;262:H1474‐81. - PubMed
Davidson 1999
-
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Safety of withdrawing inhaled nitric oxide therapy in persistent pulmonary hypertension of the newborn. Pediatrics 1999;104:231‐6. - PubMed
Dobyns 1999
-
- Dobyns EL, Griebel J, Kinsella JP, Abman SH, Accurso FJ. Infant lung function after inhaled nitric oxide therapy for persistent pulmonary hypertension of the newborn. Pediatric Pulmonology 1999;28:24‐30. - PubMed
Etches 1994
-
- Etches PC, Finer NN, Barrington KJ, Graham AJ, Chan WKY. Nitric oxide reverses pulmonary hypertension in the newborn piglet. Pediatric Research 1994;35:15‐9. - PubMed
Finer 1994
-
- Finer NN, Etches PC, Kamstra B, Tierney AJ, Peliowski A, Ryan CA. Inhaled nitric oxide in infants referred for extracorporeal membrane oxygenation: dose response. Journal of Pediatrics 1994;124:302‐8. - PubMed
Fratacci 1991
-
- Fratacci MD, Frostell CG, Chen TY, Wain JC, Robinson DR, Zapol WM. Inhaled nitric oxide ‐ A selective pulmonary vasodilator of heparin‐protamine vasoconstriction in sheep. Anesthesiology 1991;75:990‐9. - PubMed
Frostell 1991
-
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide ‐ A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991;83:2038‐47. - PubMed
GRADEpro [Computer program]
-
- McMaster University. GRADEpro [www.gradepro.org]. Ontario: McMaster University, 2014.
Greenbaum 1967
-
- Greenbaum R, Bay J, Hargreaves MD, Kain ML, Kelman GR, Nunn JF, et al. Effects of higher oxides of nitrogen on the anaesthetized dog. British Journal of Anaesthesia 1967;39:393‐404. - PubMed
Haddad 1993
-
- Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite‐induced injury to pulmonary surfactants. American Journal of Physiology 1993;265:L555‐64. - PubMed
Heal 1995
-
- Heal CA, Spencer SA. Methaemoglobinaemia with high‐dose nitric oxide administration. Acta Paediatrica 1995;84:1318‐9. - PubMed
Higenbottam 1988
-
- Higenbottam T, Pepke‐Zaba J, Scott J, Wollman P, Coutts C, Wallwork J. Inhaled "endothelium‐derived relaxing factor" (EDRF) in primary hypertension (PPH). American Review of Respiratory Disease 1988;137:A107.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hogman 1993
-
- Hogman M, Frostell C, Arnberg H, Hedenstierma G. Bleeding time prolongation and NO inhalation. Lancet 1993;341:1664‐5. - PubMed
Hogman 1994
-
- Hogman M, Frostell C, Arnberg H, Sandhagen B, Hedenstierna G. Prolonged bleeding time during nitric oxide inhalation in the rabbit. Acta Physiologica Scandinavica 1994;151:125. - PubMed
Kinsella 1992a
-
- Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. American Journal of Physiology 1992;263:H875‐80. - PubMed
Kinsella 1992b
-
- Kinsella JP, Neish SR, Shaffer E, Abman SH. Low‐dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:819‐20. - PubMed
Kinsella 1993
-
- Kinsella JP, Neish SR, Ivy DD, Shaffer E, Abman SH. Clinical responses to prolonged treatment of persistent pulmonary hypertension of the newborn with low doses of inhaled nitric oxide. Journal of Pediatrics 1993;123:103‐8. - PubMed
Lipkin 2002
-
- Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. Journal of Pediatrics 2002;140:306‐10. - PubMed
MMWR 1988
-
- National Institute for Occupational Safety and Health. NIOSH recommendations for occupational safety and health standards. MMWR 1988;37:21. - PubMed
Ninos 2000
-
- Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near‐term infants: neurodevelopmental follow‐up of the neonatal inhaled nitric oxide study group (NINOS). Journal of Pediatrics 2000;136:611‐7. - PubMed
Pepke‐Zaba 1991
-
- Pepke‐Zaba J, Higenbottam TW, Dinh‐Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilation in pulmonary hypertension. Lancet 1991;338:1173‐4. - PubMed
Radomski 1993
-
- Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thrombosis and Haemostasis 1993;70:36‐41. - PubMed
Rasmussen 1992
-
- Rasmussen TR, Kjaergaard SK, Tarp U, Pedersen OF. Delayed effects of NO2 exposure on alveolar permeability and glutathione peroxidase in healthy humans. American Review of Respiratory Disease 1992;146:654‐9. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1992
-
- Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:818‐9. - PubMed
Roberts 1993
-
- Roberts JD Jr, Chen T‐Y. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circulation Research 1993;72:246. - PubMed
Rosenberg 1997
-
- Rosenberg AA, Kennaugh JM, Moreland SG, Fashaw LM, Hale KA, Torielli FM, et al. Longitudinal follow‐up of a cohort of newborn infants treated with inhaled nitric oxide for persistent pulmonary hypertension. Journal of Pediatrics 1997;131:70‐5. - PubMed
Rossaint 1993
-
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. New England Journal of Medicine 1993;328:399‐405. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook Updated October 2013.
References to other published versions of this review
Finer 1997
-
- Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full‐term and nearly full‐term newborn infants. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000399] - DOI
Finer 1999
-
- Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full‐term and nearly full‐term newborn infants. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000399] - DOI
Finer 2001
-
- Finer NN, Barrington KJ. Nitric oxide in respiratory failure in full‐term and nearly full‐term newborn infants (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000399.pub2] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical