Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis
- PMID: 28056206
- PMCID: PMC5559888
- DOI: 10.1200/JCO.2016.67.9258
Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis
Abstract
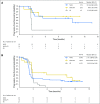
Purpose Mucosal melanoma is an aggressive malignancy with a poor response to conventional therapies. The efficacy and safety of nivolumab (a programmed death-1 checkpoint inhibitor), alone or combined with ipilimumab (a cytotoxic T-lymphocyte antigen-4 checkpoint inhibitor), have not been reported in this rare melanoma subtype. Patients and Methods Data were pooled from 889 patients who received nivolumab monotherapy in clinical studies, including phase III trials; 86 (10%) had mucosal melanoma and 665 (75%) had cutaneous melanoma. Data were also pooled for patients who received nivolumab combined with ipilimumab (n = 35, mucosal melanoma; n = 326, cutaneous melanoma). Results Among patients who received nivolumab monotherapy, median progression-free survival was 3.0 months (95% CI, 2.2 to 5.4 months) and 6.2 months (95% CI, 5.1 to 7.5 months) for mucosal and cutaneous melanoma, with objective response rates of 23.3% (95% CI, 14.8% to 33.6%) and 40.9% (95% CI, 37.1% to 44.7%), respectively. Median progression-free survival in patients treated with nivolumab combined with ipilimumab was 5.9 months (95% CI, 2.8 months to not reached) and 11.7 months (95% CI, 8.9 to 16.7 months) for mucosal and cutaneous melanoma, with objective response rates of 37.1% (95% CI, 21.5% to 55.1%) and 60.4% (95% CI, 54.9% to 65.8%), respectively. For mucosal and cutaneous melanoma, respectively, the incidence of grade 3 or 4 treatment-related adverse events was 8.1% and 12.5% for nivolumab monotherapy and 40.0% and 54.9% for combination therapy. Conclusion To our knowledge, this is the largest analysis of data for anti-programmed death-1 therapy in mucosal melanoma to date. Nivolumab combined with ipilimumab seemed to have greater efficacy than either agent alone, and although the activity was lower in mucosal melanoma, the safety profile was similar between subtypes.
Figures




References
-
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. - PubMed
-
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomized, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

