Chloroplast: the Trojan horse in plant-virus interaction
- PMID: 28056496
- PMCID: PMC6638057
- DOI: 10.1111/mpp.12533
Chloroplast: the Trojan horse in plant-virus interaction
Abstract
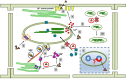
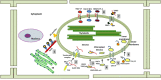
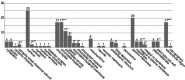
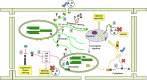
The chloroplast is one of the most dynamic organelles of a plant cell. It carries out photosynthesis, synthesizes major phytohormones, plays an active part in the defence response and is crucial for interorganelle signalling. Viruses, on the other hand, are extremely strategic in manipulating the internal environment of the host cell. The chloroplast, a prime target for viruses, undergoes enormous structural and functional damage during viral infection. Indeed, large proportions of affected gene products in a virus-infected plant are closely associated with the chloroplast and the process of photosynthesis. Although the chloroplast is deficient in gene silencing machinery, it elicits the effector-triggered immune response against viral pathogens. Virus infection induces the organelle to produce an extensive network of stromules which are involved in both viral propagation and antiviral defence. From studies over the last few decades, the involvement of the chloroplast in the regulation of plant-virus interaction has become increasingly evident. This review presents an exhaustive account of these facts, with their implications for pathogenicity. We have attempted to highlight the intricacies of chloroplast-virus interactions and to explain the existing gaps in our current knowledge, which will enable virologists to utilize chloroplast genome-based antiviral resistance in economically important crops.
Keywords: chloroplast; defence; infection; interaction; replication; translation; virus.
© 2017 BSPP AND JOHN WILEY & SONS LTD.
Conflict of interest statement
The authors declare that they do not have any conflicts of interest.
Figures
References
-
- Abbink, T.E. , Peart, J.R. , Mos, T.N. , Baulcombe, D.C. and Bol, J.F. (2002) Silencing of a gene encoding a protein component of the oxygen‐evolving complex of photosystem II enhances virus replication in plants. Virology, 295, 307–319. - PubMed
-
- Andersson, B. and Aro, E.M. (1997) Proteolytic activities and proteases of plant chloroplasts. Physiol. Plant. 100, 780–793.
-
- Ashraf, M. and Harris, P.J.C. (2013) Photosynthesis under stressful environments: an overview. Photosynthetica, 51, 163–190.
-
- Balachandran, S. , Hull, R.J. , Vaadia, Y. , Wolf, S. and Lucas, W.J. (1995) Alteration in carbon partitioning induced by the movement protein of Tobacco mosaic virus originates in the mesophyll and is independent of change in the plasmodesmal size exclusion limit. Plant Cell Environ. 18, 1301–1310.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

