Study Protocol: The Norfolk Diabetes Prevention Study [NDPS]: a 46 month multi - centre, randomised, controlled parallel group trial of a lifestyle intervention [with or without additional support from lay lifestyle mentors with Type 2 diabetes] to prevent transition to Type 2 diabetes in high risk groups with non - diabetic hyperglycaemia, or impaired fasting glucose
- PMID: 28056894
- PMCID: PMC5217324
- DOI: 10.1186/s12889-016-3929-5
Study Protocol: The Norfolk Diabetes Prevention Study [NDPS]: a 46 month multi - centre, randomised, controlled parallel group trial of a lifestyle intervention [with or without additional support from lay lifestyle mentors with Type 2 diabetes] to prevent transition to Type 2 diabetes in high risk groups with non - diabetic hyperglycaemia, or impaired fasting glucose
Abstract
Background: This 7 year NIHR programme [2011-2018] tests the primary hypothesis that the NDPS diet and physical activity intervention will reduce the risk of transition to type 2 diabetes (T2DM) in groups at high risk of Type 2 diabetes. The NDPS programme recognizes the need to reduce intervention costs through group delivery and the use of lay mentors with T2DM, the realities of normal primary care, and the complexity of the current glycaemic categorisation of T2DM risk.
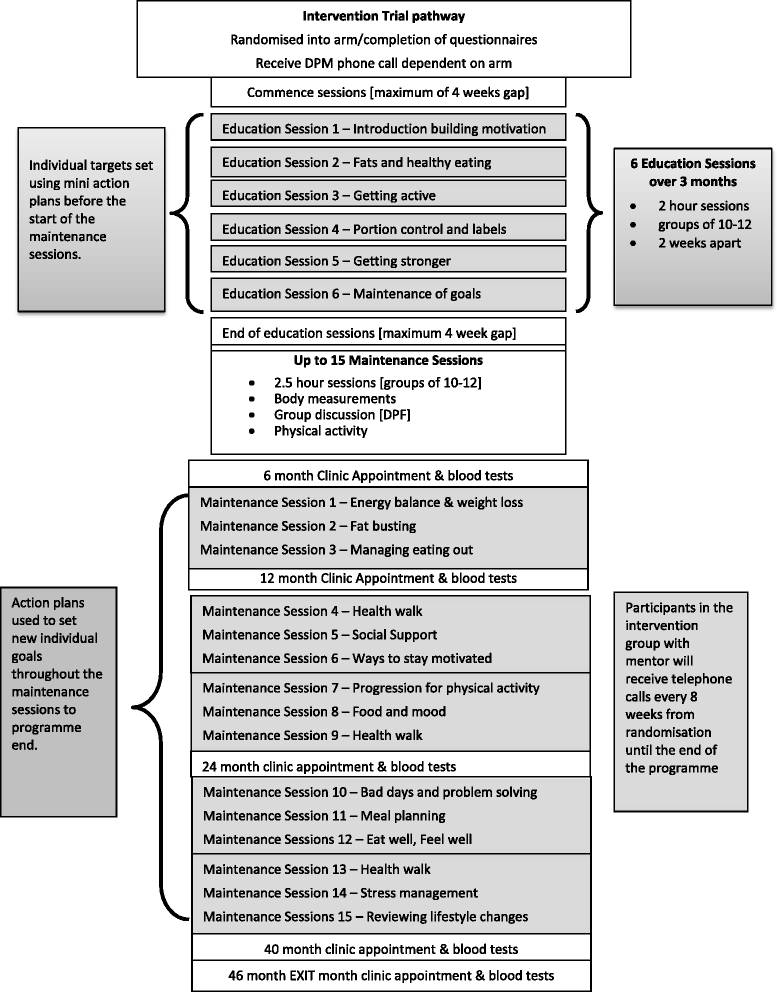
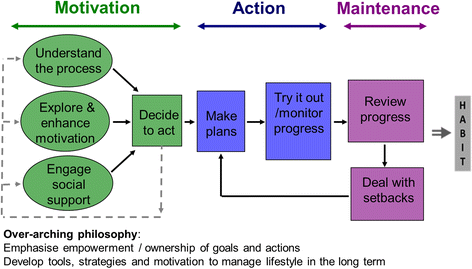
Methods: NDPS identifies people at highest risk of T2DM on the databases of 135 general practices in the East of England for further screening with ab fasting plasma glucose and glycosylated haemoglobin [HbA1c]. Those with an elevated fasting plasma glucose [impaired fasting glucose or IFG] with or without an elevated HbA1c [non -diabetic hyperglycaemia; NDH] are randomised into three treatment arms: a control arm receiving no trial intervention, an arm receiving an intensive bespoke group-based diet and physical activity intervention, and an arm receiving the same intervention with enhanced support from people with T2DM trained as diabetes prevention mentors [DPM]. The primary end point is cumulative transition rates to T2DM between the two intervention groups, and between each intervention group and the control group at 46 months. Participants with screen detected T2DM are randomized into an equivalent prospective controlled trial with the same intervention and control arms with glycaemic control [HbA1c] at 46 months as the primary end point. Participants with NDH and a normal fasting plasma glucose are randomised into an equivalent prospective controlled intervention trial with follow up for 40 months. The intervention comprises six education sessions for the first 12 weeks and then up to 15 maintenance sessions until intervention end, all delivered in groups, with additional support from a DPM in one treatment arm.
Discussion: The NDPS programme reports in 2018 and will provide trial outcome data for a group delivered diabetes prevention intervention, supported by lay mentors with T2DM, with intervention in multiple at risk glycaemic categories, and that takes into account the realities of normal clinical practice.
Trial registration: ISRCTN34805606 (Retrospectively registered 16.3.16).
Keywords: Diabetes prevention; Impaired fasting glucose; Lay mentors; Lifestyle intervention; Non diabetic hyperglycaemia; Type 2 diabetes.
Figures
References
-
- Comment. Reducing global diabetes burden by implementing solutions and identifying gaps: a Lancet Commission. Lancet. 2016; 387:1494–95 - PubMed
-
- Schwarz PE, Greaves CE, Lindstrom J, Yates T, Davies MJ. Nonpharmacological interventions for the prevention of Type 2 diabetes. Nat Rev Endocrinol. 2012;8:363–373. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical