The main rhinovirus respiratory tract adhesion site (ICAM-1) is upregulated in smokers and patients with chronic airflow limitation (CAL)
- PMID: 28056984
- PMCID: PMC5217320
- DOI: 10.1186/s12931-016-0483-8
The main rhinovirus respiratory tract adhesion site (ICAM-1) is upregulated in smokers and patients with chronic airflow limitation (CAL)
Abstract
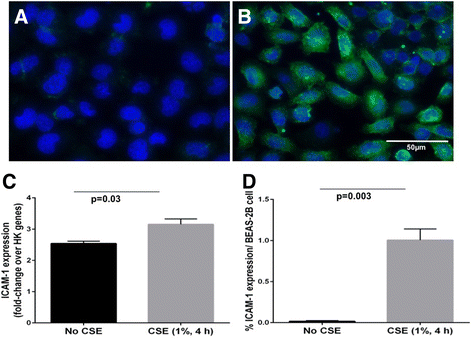
Background: ICAM-1 is a major receptor for ~60% of human rhinoviruses, and non-typeable Haemophilus influenzae, two major pathogens in COPD. Increased cell-surface expression of ICAM-1 in response to tobacco smoke exposure has been suggested. We have investigated epithelial ICAM-1 expression in both the large and small airways, and lung parenchyma in smoking-related chronic airflow limitation (CAL) patients.
Methods: We evaluated epithelial ICAM-1 expression in resected lung tissue: 8 smokers with normal spirometry (NLFS); 29 CAL patients (10 small-airway disease; 9 COPD-smokers; 10 COPD ex-smokers); Controls (NC): 15 normal airway/lung tissues. Immunostaining with anti-ICAM-1 monoclonal antibody was quantified with computerized image analysis. The percent and type of cells expressing ICAM-1 in large and small airway epithelium and parenchyma were enumerated, plus percentage of epithelial goblet and submucosal glands positive for ICAM- 1.
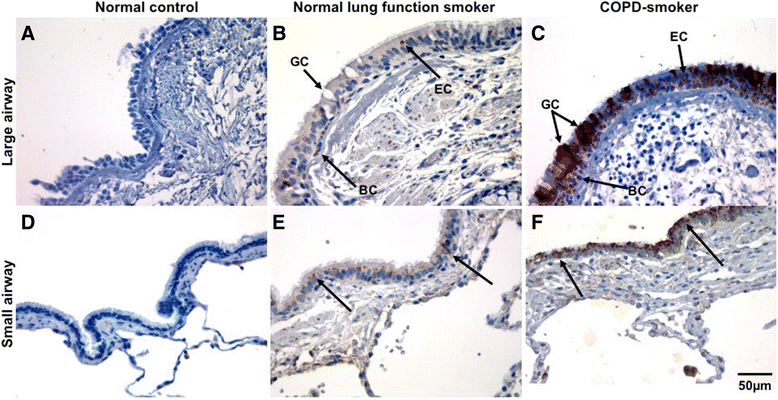
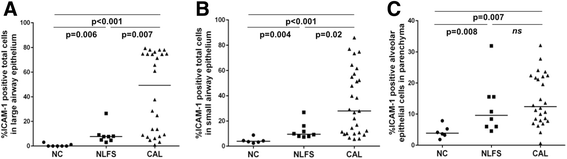
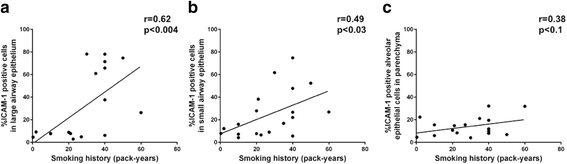
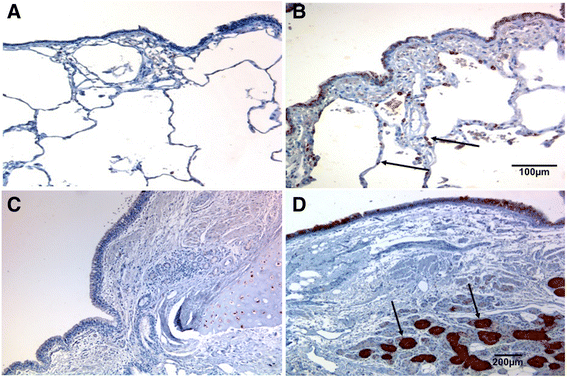
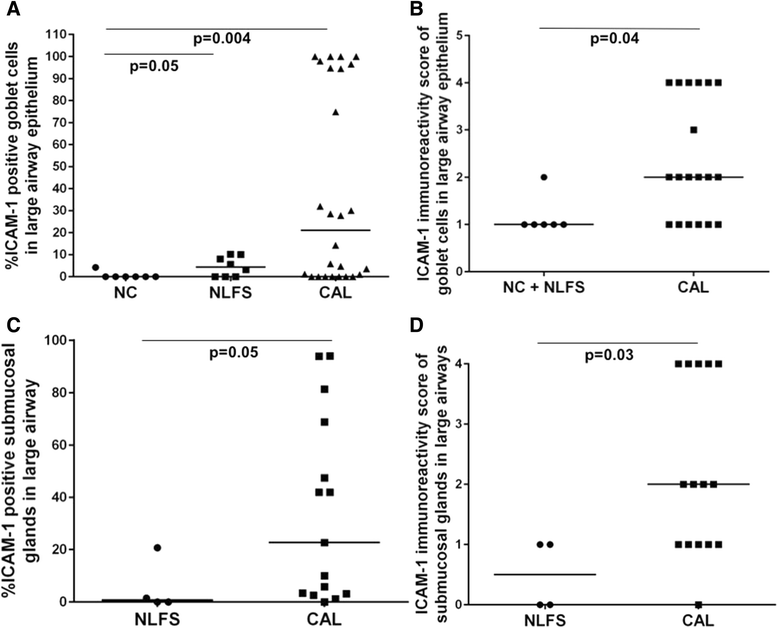
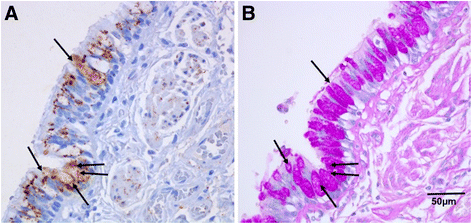
Results: A major increase in ICAM-1 expression in epithelial cells was found in both large (p < 0.006) and small airways (p < 0.004) of CAL subjects compared to NC, with NLFS being intermediate. In the CAL group, both basal and luminal areas stained heavily for ICAM-1, so did goblet cells and sub-mucosal glands, however in either NC or NLFS subjects, only epithelial cell luminal surfaces stained. ICAM-1 expression on alveolar pneumocytes (mainly type II) was slightly increased in CAL and NLFS (p < 0.01). Pack-years of smoking correlated with ICAM-1 expression (r = 0.49; p < 0.03).
Conclusion: Airway ICAM-1 expression is markedly upregulated in CAL group, which could be crucial in rhinoviral and NTHi infections. The parenchymal ICAM-1 is affected by smoking, with no further enhancement in CAL subjects.
Keywords: Chronic airflow limitation; Chronic obstructive pulmonary disease; Epithelial adhesion; Human rhinovirus; Intercellular adhesion molecule-1.
Figures
References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). News & events from around the world, 2015. Available from http://www.goldcopd.org/. [Accessed 5 Jan 2015].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

